当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Relationship between Protein-Induced Membrane Curvature and Membrane Thermal Undulation
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2024-01-05 , DOI: 10.1021/acs.jpcb.3c06775 Xiangyuan Li 1 , Lei Fu 1 , Shan Zhang 1 , Yi Dong 1 , Lianghui Gao 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2024-01-05 , DOI: 10.1021/acs.jpcb.3c06775 Xiangyuan Li 1 , Lei Fu 1 , Shan Zhang 1 , Yi Dong 1 , Lianghui Gao 1
Affiliation
|
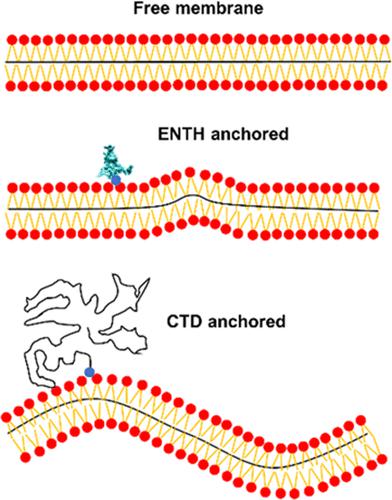
This work studied the membrane curvature generated by anchored proteins lacking amphipathic helices and intrinsic morphologies, including the Epsin N-terminal homology domain, intrinsically disordered C-terminal domain, and truncated C-terminal fragments, by using coarse-grained molecular dynamics simulations. We found that anchored proteins can stabilize the thermal undulation of membranes at a wavelength five times the protein’s binding size. This proportional connection is governed by the membrane bending rigidity and protein density. Extended intrinsically disordered proteins with relatively high hydrophobicity favor colliding with the membrane, leading to a much larger binding size, and show superiority in generating membrane curvature at low density over folded proteins.
中文翻译:

蛋白质诱导的膜曲率与膜热波动的关系
这项工作通过使用粗粒度分子动力学模拟研究了缺乏两亲性螺旋和内在形态的锚定蛋白产生的膜曲率,包括 Epsin N 末端同源结构域、内在无序的 C 末端结构域和截短的 C 末端片段。我们发现锚定蛋白可以在蛋白质结合大小的五倍波长下稳定膜的热波动。这种比例连接受膜弯曲刚度和蛋白质密度的约束。具有相对高疏水性的延伸的内在无序蛋白有利于与膜碰撞,导致更大的结合尺寸,并且在以低密度产生膜曲率方面显示出优于折叠蛋白的优点。
更新日期:2024-01-05
中文翻译:

蛋白质诱导的膜曲率与膜热波动的关系
这项工作通过使用粗粒度分子动力学模拟研究了缺乏两亲性螺旋和内在形态的锚定蛋白产生的膜曲率,包括 Epsin N 末端同源结构域、内在无序的 C 末端结构域和截短的 C 末端片段。我们发现锚定蛋白可以在蛋白质结合大小的五倍波长下稳定膜的热波动。这种比例连接受膜弯曲刚度和蛋白质密度的约束。具有相对高疏水性的延伸的内在无序蛋白有利于与膜碰撞,导致更大的结合尺寸,并且在以低密度产生膜曲率方面显示出优于折叠蛋白的优点。































 京公网安备 11010802027423号
京公网安备 11010802027423号