当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
De-epimerization of Monosaccharides by Dynamic Kinetic Resolution: Ligand-Controlled Synthesis of O-Aryl-Glycosides through Copper-Catalyzed Cross-Coupling
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-01-05 , DOI: 10.1002/adsc.202300997
Tian-Yang Hou Liu 1 , Yi-Xian Li 1 , Yue-Mei Jia 1 , Chu-Yi Yu 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-01-05 , DOI: 10.1002/adsc.202300997
Tian-Yang Hou Liu 1 , Yi-Xian Li 1 , Yue-Mei Jia 1 , Chu-Yi Yu 2
Affiliation
|
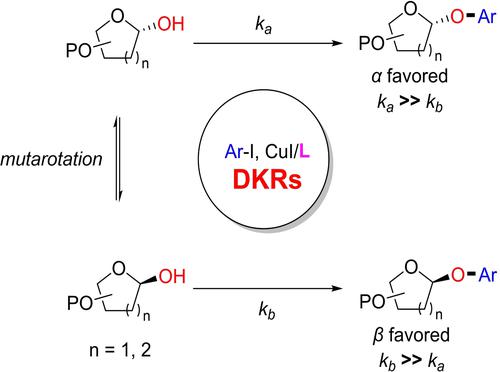
We report a strategy for stereoselective O-aryl-glycoside synthesis by copper-catalyzed cross-coupling of a variety of anomeric sugars and (hetero)aromatic iodides. Stereocontrol of the α/β selectivity can be successfully realized by slight structural modifications of the oxalic diamide ligands. Mechanistic studies indicated a dynamic kinetic resolution (DKR) reaction mechanism controlled by the ligand structures. This reaction could be performed on gram scale, and has also been applied to the synthesis of some natural products.
中文翻译:

通过动态动力学拆分单糖的去差向异构化:通过铜催化交叉偶联配体控制合成 O-芳基糖苷
我们报告了一种通过铜催化交叉偶联各种异头糖和(杂)芳香族碘化物来立体选择性 O-芳基糖苷合成的策略。通过草酸二酰胺配体的轻微结构修饰可以成功实现α/β选择性的立体控制。机理研究表明了由配体结构控制的动态动力学拆分(DKR)反应机制。该反应可以在克级进行,并且也已应用于一些天然产物的合成。
更新日期:2024-01-05
中文翻译:

通过动态动力学拆分单糖的去差向异构化:通过铜催化交叉偶联配体控制合成 O-芳基糖苷
我们报告了一种通过铜催化交叉偶联各种异头糖和(杂)芳香族碘化物来立体选择性 O-芳基糖苷合成的策略。通过草酸二酰胺配体的轻微结构修饰可以成功实现α/β选择性的立体控制。机理研究表明了由配体结构控制的动态动力学拆分(DKR)反应机制。该反应可以在克级进行,并且也已应用于一些天然产物的合成。































 京公网安备 11010802027423号
京公网安备 11010802027423号