Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Orthogonal Deprotection Strategy of Fmoc Provides Improved Synthesis of Sensitive Peptides: Application to Z-Arg-Lys-AOMK
ACS Omega ( IF 3.7 ) Pub Date : 2024-01-05 , DOI: 10.1021/acsomega.3c08629
Jehad Almaliti 1, 2 , Momen Alhindy 2 , Michael C Yoon 3 , Vivian Hook 3, 4 , Tadeusz F Molinski 2, 3, 5 , Anthony J O'Donoghue 3 , William H Gerwick 2, 3
ACS Omega ( IF 3.7 ) Pub Date : 2024-01-05 , DOI: 10.1021/acsomega.3c08629
Jehad Almaliti 1, 2 , Momen Alhindy 2 , Michael C Yoon 3 , Vivian Hook 3, 4 , Tadeusz F Molinski 2, 3, 5 , Anthony J O'Donoghue 3 , William H Gerwick 2, 3
Affiliation
|
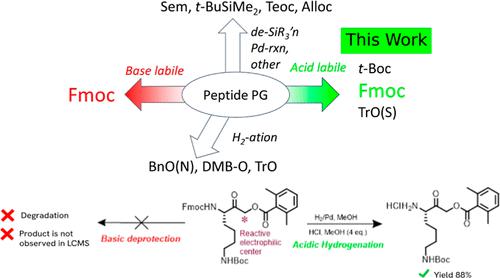
Protecting groups (PGs) in peptide synthesis have inspired advanced design principles that incorporate “orthogonality” for selective C- and N-terminus and side-chain deprotections. The conventionally acid-stable 9-fluorenylmethoxycarbonyl (Fmoc) group is one of the most widely used N-protection groups in solid- and solution-phase synthesis. Despite the versatility of Fmoc, deprotection by the removal of the Fmoc group to unmask primary amines requires the use of a basic secondary amine nucleophile, but this stratagem poses challenges in sensitive molecules that bear reactive electrophilic groups. An expansion of PG versatility, a tunable orthogonality, in the late-stage synthesis of peptides would add flexibility to the synthetic design and implementation. Here, we report a novel Fmoc deprotection method using hydrogenolysis under mildly acidic conditions for the synthesis of Z-Arg-Lys-acyloxymethyl ketone (Z-R-K-AOMK). This new method is not only valuable for Fmoc deprotection in the synthesis of complex peptides that contain highly reactive electrophiles, or other similar sensitive functional groups, that are incompatible with traditional Fmoc deprotection conditions but also tolerant of N-Boc groups present in the substrate.
中文翻译:

Fmoc 的正交脱保护策略改善了敏感肽的合成:在 z-arg-lys-AOMK 中的应用
肽合成中的保护基团 (PG) 激发了先进的设计原则,这些原则结合了选择性 C 端和 N 端以及侧链脱保护的“正交性”。常规的酸稳定的 9-芴基甲氧基羰基 (Fmoc) 基团是固相和液相合成中使用最广泛的 N 保护基团之一。尽管 Fmoc 用途广泛,但通过去除 Fmoc 基团以揭示伯胺而脱保护需要使用碱性仲胺亲核试剂,但这种策略对带有反应性亲电基团的敏感分子构成了挑战。在肽的后期合成中,PG 多功能性的扩展,即可调的正交性,将增加合成设计和实施的灵活性。在这里,我们报道了一种新的 Fmoc 脱保护方法,该方法在弱酸性条件下使用氢解合成 Z-Arg-Lys-酰氧基甲基酮 (Z-R-K-AOMK)。这种新方法不仅对合成含有高反应性亲电试剂或其他类似敏感官能团的复杂肽的 Fmoc 脱保护很有价值,这些官能团与传统的 Fmoc 脱保护条件不相容,而且对底物中存在的 N-Boc 基团具有耐受性。
更新日期:2024-01-05
中文翻译:

Fmoc 的正交脱保护策略改善了敏感肽的合成:在 z-arg-lys-AOMK 中的应用
肽合成中的保护基团 (PG) 激发了先进的设计原则,这些原则结合了选择性 C 端和 N 端以及侧链脱保护的“正交性”。常规的酸稳定的 9-芴基甲氧基羰基 (Fmoc) 基团是固相和液相合成中使用最广泛的 N 保护基团之一。尽管 Fmoc 用途广泛,但通过去除 Fmoc 基团以揭示伯胺而脱保护需要使用碱性仲胺亲核试剂,但这种策略对带有反应性亲电基团的敏感分子构成了挑战。在肽的后期合成中,PG 多功能性的扩展,即可调的正交性,将增加合成设计和实施的灵活性。在这里,我们报道了一种新的 Fmoc 脱保护方法,该方法在弱酸性条件下使用氢解合成 Z-Arg-Lys-酰氧基甲基酮 (Z-R-K-AOMK)。这种新方法不仅对合成含有高反应性亲电试剂或其他类似敏感官能团的复杂肽的 Fmoc 脱保护很有价值,这些官能团与传统的 Fmoc 脱保护条件不相容,而且对底物中存在的 N-Boc 基团具有耐受性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号