Journal of Cleaner Production ( IF 9.7 ) Pub Date : 2024-01-03 , DOI: 10.1016/j.jclepro.2024.140590 Zhi Ying , Aoli Yang , Muyang Zhao , Jingyang Yang , Xiaoyuan Zheng , Binlin Dou , Guomin Cui
|
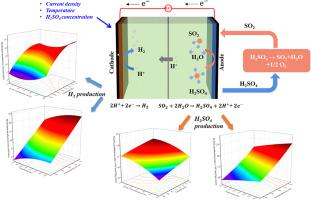
SO2-depolarized electrolysis (SDE) is the key process in the hybrid sulfur cycle for green hydrogen production, but the detailed SDE characteristics including products generation, current efficiency variation, and side reactions are unclear. Herein, a continuous SDE process was experimentally conducted and theoretically modelled. The H2 and H2SO4 production was accelerated and the current efficiency for both electrodes was improved with a proper increase in current density (50−100 mA/cm2) or temperature (293−318 K), but further increasing current density to 150 mA/cm2 or temperature to 333 K led to a negative trend because of the anode corrosion or declined SO2 solubility. The increasing initial H2SO4 concentration (10−40 wt%) facilitated the cathodic H2 production and current efficiency, while negatively influenced the anodic SO2 conversion. The parasitic reactions occurred at cathode consumed H2 and resulted to the cathodic current efficiency well below 100%. A rigorous mathematical regression model correlating the production rates of H2 and H2SO4 with current density, temperature, and initial H2SO4 concentration was developed, and combined with the analysis of variance, current density was suggested the biggest factor affecting products generation in SDE process. These findings demonstrate a practical avenue for efficient SO2 conversion and H2 production in SDE process of hybrid sulfur cycle.
中文翻译:

混合硫循环连续SO2去极化电解制氢实验研究与建模
SO 2去极化电解(SDE)是混合硫循环绿色制氢的关键过程,但SDE的详细特征,包括产物生成、电流效率变化和副反应尚不清楚。在此,对连续 SDE 过程进行了实验和理论建模。随着电流密度(50−100 mA/cm 2)或温度(293−318 K)的适当增加, H 2和H 2 SO 4的产生加速,并且两个电极的电流效率都得到提高,但进一步增加电流密度由于阳极腐蚀或SO 2溶解度下降,电流达到150 mA/cm 2或温度达到333 K 会导致负趋势。增加初始H 2 SO 4浓度(10−40 wt%)有利于阴极H 2 的产生和电流效率,同时对阳极SO 2转化产生负面影响。阴极发生寄生反应,消耗H 2,导致阴极电流效率远低于100%。建立了严格的数学回归模型,将H 2和H 2 SO 4的产率与电流密度、温度和初始H 2 SO 4浓度相关联,并结合方差分析,提出了电流密度是影响产品的最大因素。 SDE过程中的生成。这些发现证明了在混合硫循环的SDE过程中高效SO 2转化和H 2生产的实用途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号