Synlett ( IF 1.7 ) Pub Date : 2024-01-04 , DOI: 10.1055/a-2219-5767 Shunki Jinno 1 , Tomoko Kawasaki-Takasuka 2 , Keiji Mori 1
|
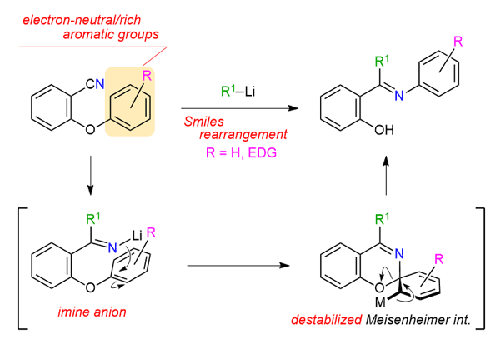
We have revisited the imine-anion-mediated Smiles rearrangement for the synthesis of ortho-hydroxyphenyl arylketimines. Detailed examinations revealed that migration of various aromatic groups, previously considered to be unsuited to SNAr-type reactions, such as electron-rich or sterically hindered aromatic groups, can be accomplished by introducing bulky 9-anthryllithium as a nucleophile. Among the aromatic groups examined, naphthyl groups (1- and 2-naphthyl groups) exhibited an excellent performance, and their migration ability was well illustrated by the reaction with less bulky nucleophiles.
中文翻译:

亚胺阴离子介导的微笑重排的更新:在电子中性/富芳香族基团迁移中的应用
我们重新审视了亚胺阴离子介导的 Smiles 重排,用于合成邻羟基苯基芳基酮亚胺。详细的研究表明,以前被认为不适合SN Ar型反应的各种芳香族基团的迁移,例如富电子或空间位阻的芳香族基团,可以通过引入大体积的9-蒽基锂作为亲核试剂来实现。在所检测的芳香族基团中,萘基(1-萘基和2-萘基)表现出优异的性能,并且通过与体积较小的亲核试剂的反应很好地说明了它们的迁移能力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号