当前位置:
X-MOL 学术
›
Chemosphere
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The mechanism of photodegradation reaction of different dissociation forms of tetrabromobisphenol S in water with free radicals and the ecotoxicity evaluation of related products
Chemosphere ( IF 8.1 ) Pub Date : 2024-01-04 , DOI: 10.1016/j.chemosphere.2024.141136 Ying Lu 1 , Se Wang 1
Chemosphere ( IF 8.1 ) Pub Date : 2024-01-04 , DOI: 10.1016/j.chemosphere.2024.141136 Ying Lu 1 , Se Wang 1
Affiliation
|
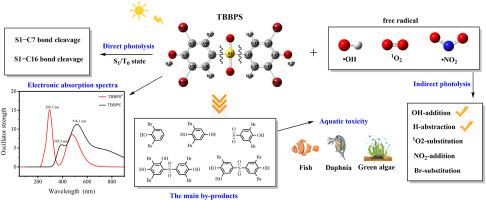
Tetrabromobisphenol S (TBBPS) is a widely used brominated flame retardant that has attracted environmental concern due to its abundant presence in water. The objective of this study is to systematically analyze the direct photolysis and degradation mechanisms of TBBPS in two different dissociation forms in water, as well as to evaluate their toxicological effects induced by •OH, O, and •NO radicals. The degradation mechanism of TBBPS is investigated with density functional theory (DFT) and time-dependent density functional theory (TDDFT) methods, and the toxicity of the degradation products is assessed through toxicological studies. The results of the study indicate that the OH-addition and H-abstraction reactions are favorable pathways for •OH-induced TBBPS degradation. The H-abstraction reaction of TBBPS with •OH was more favorable than the •OH addition reaction. However, in the degradation of TBBPS, the •OH addition reaction was favored over the H-abstraction reaction. Additionally, the indirect photolysis of TBBPS by O and •NO in water was found to be easier for TBBPS compared to TBBPS, with degradation mechanisms involving Br-substitution and NO-addition reactions. The higher values calculated indicate that the degradation of TBBPS by O and •NO in water has been a secondary reaction. The direct photolysis reaction pathway of TBBPS in water has involved the cleavage of the S1–C7 and S1–C16 bonds. For TBBPS in the S/T states, the primary reaction pathway is the cleavage of the S1–C16 bond, while for TBBPS, the primary reaction pathway is the cleavage of the S1–C7 bond. Furthermore, the computational toxicology results indicate a slight increase in the toxicity levels of most products, highlighting the significance of investigating the degradation byproducts of TBBPS in greater detail.
中文翻译:

水中不同解离形式的四溴双酚S与自由基的光降解反应机理及相关产品的生态毒性评价
四溴双酚 S (TBBPS) 是一种广泛使用的溴化阻燃剂,由于其在水中的大量存在而引起了环境问题。本研究的目的是系统分析TBBPS在水中两种不同解离形式的直接光解和降解机制,并评估其由•OH、O和•NO自由基诱导的毒理学效应。采用密度泛函理论(DFT)和时间相关密度泛函理论(TDDFT)方法研究了TBBPS的降解机理,并通过毒理学研究评估了降解产物的毒性。研究结果表明,OH-加成反应和H-夺取反应是·OH诱导的TBBPS降解的有利途径。 TBBPS与·OH的夺氢反应比·OH加成反应更有利。然而,在TBBPS的降解过程中,·OH加成反应优于H-夺取反应。此外,与TBBPS相比,水中的O和NO对TBBPS的间接光解被发现更容易,降解机制涉及Br取代和NO加成反应。较高的计算值表明水中的 O 和 NO 降解 TBBPS 是二次反应。 TBBPS 在水中的直接光解反应途径涉及 S1-C7 和 S1-C16 键的断裂。对于处于S/T状态的TBBPS,主要反应途径是S1-C16键的断裂,而对于TBBPS,主要反应途径是S1-C7键的断裂。 此外,计算毒理学结果表明大多数产品的毒性水平略有增加,凸显了更详细地研究 TBBPS 降解副产物的重要性。
更新日期:2024-01-04
中文翻译:

水中不同解离形式的四溴双酚S与自由基的光降解反应机理及相关产品的生态毒性评价
四溴双酚 S (TBBPS) 是一种广泛使用的溴化阻燃剂,由于其在水中的大量存在而引起了环境问题。本研究的目的是系统分析TBBPS在水中两种不同解离形式的直接光解和降解机制,并评估其由•OH、O和•NO自由基诱导的毒理学效应。采用密度泛函理论(DFT)和时间相关密度泛函理论(TDDFT)方法研究了TBBPS的降解机理,并通过毒理学研究评估了降解产物的毒性。研究结果表明,OH-加成反应和H-夺取反应是·OH诱导的TBBPS降解的有利途径。 TBBPS与·OH的夺氢反应比·OH加成反应更有利。然而,在TBBPS的降解过程中,·OH加成反应优于H-夺取反应。此外,与TBBPS相比,水中的O和NO对TBBPS的间接光解被发现更容易,降解机制涉及Br取代和NO加成反应。较高的计算值表明水中的 O 和 NO 降解 TBBPS 是二次反应。 TBBPS 在水中的直接光解反应途径涉及 S1-C7 和 S1-C16 键的断裂。对于处于S/T状态的TBBPS,主要反应途径是S1-C16键的断裂,而对于TBBPS,主要反应途径是S1-C7键的断裂。 此外,计算毒理学结果表明大多数产品的毒性水平略有增加,凸显了更详细地研究 TBBPS 降解副产物的重要性。































 京公网安备 11010802027423号
京公网安备 11010802027423号