当前位置:
X-MOL 学术
›
Chemosphere
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of cationic surfactant modified two-dimensional (2D) multi-layered Ti3C2Tx MXene for methyl orange removal from aqueous solution: Kinetic, equilibrium, and adsorption mechanisms
Chemosphere ( IF 8.1 ) Pub Date : 2024-01-03 , DOI: 10.1016/j.chemosphere.2023.141058 Pouya Najibikhah 1 , Ahmad Rahbar-Kelishami 1
Chemosphere ( IF 8.1 ) Pub Date : 2024-01-03 , DOI: 10.1016/j.chemosphere.2023.141058 Pouya Najibikhah 1 , Ahmad Rahbar-Kelishami 1
Affiliation
|
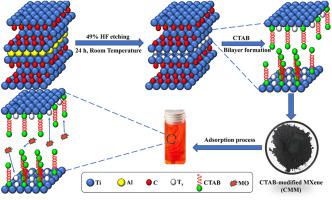
In this study, cetyltrimethylammonium bromide-modified multi-layered TiCT MXene (CMM) was produced using a TiAlC precursor, and its capacity to remove the anionic dye, methyl orange (MO), was investigated in detail. An electrostatic combination between negatively charged TiCT nanosheets and cationic surfactant solution (CTAB) produced this adsorbent. This triggered an exposure of the accessible active sites to further boost adsorption effectiveness by increasing the distance between the MXene nanosheets. Prepared adsorbents were characterized using some analytical techniques, including TGA, FESEM, EDX, FTIR, XRD, and N adsorption-desorption. Furthermore, some influencing parameters such as contact time, solution of pH, loading adsorbent, and initial dye concentration were evaluated, with findings showing that MO could adsorb CMM to its maximum capacity at an adsorbent dosage of 0.83 g/L, a contact time of 90 min, and a solution pH of 3. Adsorption results were found to be highly linked with both Langmuir isotherm (R = 0.9990) and the pseudo-second-order kinetic model (R = 0.9924). The maximum adsorption capacity of MO was obtained at approximately 213.00 mg/g. Also, hydrogen bonding, π-cation interactions, and electrostatic adsorption can all be implicated in the mechanism of MO adsorption on CMM. The fabricated CMM is presented as a prospective adsorbent for the removal of dyes from polluted water, demonstrating robust recyclability for up to the fifth iteration. All these outstanding properties indicate that cetyltrimethylammonium bromide-modified multi-layered TiCT MXene can be considered as applicable adsorbents for textile pollutants.
中文翻译:

阳离子表面活性剂改性二维 (2D) 多层 Ti3C2TX MXene 的制备用于从水溶液中去除甲基橙:动力学、平衡和吸附机制
在本研究中,使用 TiAlC 前驱体生产十六烷基三甲基溴化铵改性的多层 TiCT MXene (CMM),并详细研究了其去除阴离子染料甲基橙 (MO) 的能力。带负电的 TiCT 纳米片和阳离子表面活性剂溶液 (CTAB) 之间的静电结合产生了这种吸附剂。这触发了可接近的活性位点的暴露,通过增加 MXene 纳米片之间的距离来进一步提高吸附效率。使用一些分析技术对制备的吸附剂进行表征,包括 TGA、FESEM、EDX、FTIR、XRD 和 N 吸附-解吸。此外,还评估了一些影响参数,如接触时间、溶液pH值、吸附剂负载和初始染料浓度,结果表明,在吸附剂用量为0.83 g/L、接触时间为90 分钟,溶液 pH 为 3。发现吸附结果与 Langmuir 等温线 (R = 0.9990) 和准二级动力学模型 (R = 0.9924) 高度相关。 MO 的最大吸附容量约为 213.00 mg/g。此外,氢键、π-阳离子相互作用和静电吸附都可能与 CMM 上 MO 吸附的机制有关。所制造的 CMM 被认为是一种用于去除污水中染料的前瞻性吸附剂,在第五次迭代中表现出强大的可回收性。所有这些优异的性能表明,十六烷基三甲基溴化铵改性的多层 TiCT MXene 可以被认为是适用于纺织污染物的吸附剂。
更新日期:2024-01-03
中文翻译:

阳离子表面活性剂改性二维 (2D) 多层 Ti3C2TX MXene 的制备用于从水溶液中去除甲基橙:动力学、平衡和吸附机制
在本研究中,使用 TiAlC 前驱体生产十六烷基三甲基溴化铵改性的多层 TiCT MXene (CMM),并详细研究了其去除阴离子染料甲基橙 (MO) 的能力。带负电的 TiCT 纳米片和阳离子表面活性剂溶液 (CTAB) 之间的静电结合产生了这种吸附剂。这触发了可接近的活性位点的暴露,通过增加 MXene 纳米片之间的距离来进一步提高吸附效率。使用一些分析技术对制备的吸附剂进行表征,包括 TGA、FESEM、EDX、FTIR、XRD 和 N 吸附-解吸。此外,还评估了一些影响参数,如接触时间、溶液pH值、吸附剂负载和初始染料浓度,结果表明,在吸附剂用量为0.83 g/L、接触时间为90 分钟,溶液 pH 为 3。发现吸附结果与 Langmuir 等温线 (R = 0.9990) 和准二级动力学模型 (R = 0.9924) 高度相关。 MO 的最大吸附容量约为 213.00 mg/g。此外,氢键、π-阳离子相互作用和静电吸附都可能与 CMM 上 MO 吸附的机制有关。所制造的 CMM 被认为是一种用于去除污水中染料的前瞻性吸附剂,在第五次迭代中表现出强大的可回收性。所有这些优异的性能表明,十六烷基三甲基溴化铵改性的多层 TiCT MXene 可以被认为是适用于纺织污染物的吸附剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号