当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pseudo Four-Component Synthesis of 5,6-Dihydroindolo[2,1-a]isoquinolines
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-01-04 , DOI: 10.1021/acs.joc.3c02133 Almira R Miftyakhova 1 , Tatiana N Borisova 1 , Artem N Fakhrutdinov 2 , Valentina V Ilyushenkova 2 , Alexander A Titov 1 , Ilya V Efimov 1 , Victor A Tafeenko 3 , Alexey V Varlamov 1 , Leonid G Voskressensky 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-01-04 , DOI: 10.1021/acs.joc.3c02133 Almira R Miftyakhova 1 , Tatiana N Borisova 1 , Artem N Fakhrutdinov 2 , Valentina V Ilyushenkova 2 , Alexander A Titov 1 , Ilya V Efimov 1 , Victor A Tafeenko 3 , Alexey V Varlamov 1 , Leonid G Voskressensky 1
Affiliation
|
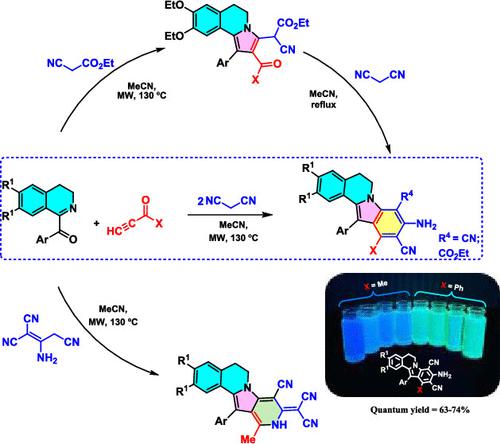
An easy synthesis of novel highly functionalized 5,6-dihydroindolo[2,1-a]isoquinolines was developed via a pseudo four-component domino reaction of 1-aroyl-3,4-dihydroisoquinolines, terminal α,β-ynones, and malononitrile. The selective formation of this biologically relevant heterocyclic core was achieved using a one-pot approach under microwave irradiation. The formation of the same skeleton through the reaction of 5,6-dihydropyrrolo[2,1-a]isoquinolines with malonic acid dinitrile supports the proposed mechanism, involving the intermediate product of the three-component reaction. Furthermore, the disproval of an alternative reaction pathway, which involved the dimerization of malononitrile followed by three-component transformation, was demonstrated. Introducing the malononitrile dimer as a CH acid resulted in the formation of a different pyrido[3′,4′:4,5]pyrrolo[2,1-a]isoquinoline core. Additionally, the synthesized 5,6-dihydroindolo[2,1-a]isoquinolines were examined for their photophysical properties, revealing their attractive luminescent characteristics.
中文翻译:

5,6-二氢吲哚并[2,1-a]异喹啉的拟四组分合成
通过1-芳酰基-3,4-二氢异喹啉、末端α,β-炔酮和丙二腈的伪四组分多米诺反应,开发了一种新型高功能化5,6-二氢吲哚并[2,1 -a ]异喹啉的简单合成方法。这种与生物学相关的杂环核心的选择性形成是在微波辐射下使用一锅法实现的。通过5,6-二氢吡咯并[2,1- a ]异喹啉与丙二酸二腈反应形成相同的骨架支持了所提出的机制,涉及三组分反应的中间产物。此外,还证明了另一种反应途径的反驳,该途径涉及丙二腈的二聚化,然后进行三组分转化。引入丙二腈二聚体作为CH酸导致形成不同的吡啶并[3',4':4,5]吡咯并[2,1- a ]异喹啉核心。此外,还检查了合成的 5,6-二氢吲哚并[2,1 -a ]异喹啉的光物理性质,揭示了其引人注目的发光特性。
更新日期:2024-01-04
中文翻译:

5,6-二氢吲哚并[2,1-a]异喹啉的拟四组分合成
通过1-芳酰基-3,4-二氢异喹啉、末端α,β-炔酮和丙二腈的伪四组分多米诺反应,开发了一种新型高功能化5,6-二氢吲哚并[2,1 -a ]异喹啉的简单合成方法。这种与生物学相关的杂环核心的选择性形成是在微波辐射下使用一锅法实现的。通过5,6-二氢吡咯并[2,1- a ]异喹啉与丙二酸二腈反应形成相同的骨架支持了所提出的机制,涉及三组分反应的中间产物。此外,还证明了另一种反应途径的反驳,该途径涉及丙二腈的二聚化,然后进行三组分转化。引入丙二腈二聚体作为CH酸导致形成不同的吡啶并[3',4':4,5]吡咯并[2,1- a ]异喹啉核心。此外,还检查了合成的 5,6-二氢吲哚并[2,1 -a ]异喹啉的光物理性质,揭示了其引人注目的发光特性。































 京公网安备 11010802027423号
京公网安备 11010802027423号