当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Early Process Development of CH7057288, a Benzofuran-Containing Selective NTRK Inhibitor
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-01-03 , DOI: 10.1021/acs.oprd.3c00434 Tomohiro Oki 1 , Junichi Shiina 2 , Hiroshi Fukuda 2 , Minoru Yamawaki 2 , Yasushi Kito 2 , Masaki Tomizawa 2 , Hiroshi Iwamura 1 , Masao Tsukazaki 1 , Azusa Toya 1 , Shun Tsuzaki 1 , Naoto Hama 3 , Akira Kawase 1 , Kenichi Nomura 2 , Hisashi Ito 3 , Kenji Maeda 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-01-03 , DOI: 10.1021/acs.oprd.3c00434 Tomohiro Oki 1 , Junichi Shiina 2 , Hiroshi Fukuda 2 , Minoru Yamawaki 2 , Yasushi Kito 2 , Masaki Tomizawa 2 , Hiroshi Iwamura 1 , Masao Tsukazaki 1 , Azusa Toya 1 , Shun Tsuzaki 1 , Naoto Hama 3 , Akira Kawase 1 , Kenichi Nomura 2 , Hisashi Ito 3 , Kenji Maeda 1
Affiliation
|
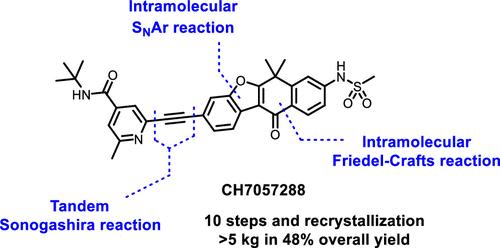
This work describes the development of a scalable manufacturing process for CH7057288, a neurotrophic tyrosine receptor kinase (NTRK) inhibitor that selectively targets NTRK positive cancers. NTRK fusions are extremely attractive and promising therapeutic targets, and in order to deliver CH7057288 as a new treatment option for patients, it is crucial to be able to rapidly supply a large amount of this innovative drug for GLP toxicology studies and first-in-human (FIH) studies. By employing an 11-step process, we have been able to synthesize CH7057288 from methyl 2-(3-aminophenyl)-2-methylpropanoate with high purity and in excellent yield. The distinguishing features of this process include sequential double cyclization, leading to four-membered benzofuran-fused heterocycles. By employing this process, we successfully produced a total of 5.5 kg of CH7057288.
中文翻译:

CH7057288(一种含苯并呋喃的选择性 NTRK 抑制剂)的早期工艺开发
这项工作描述了 CH7057288 的可扩展制造工艺的开发,CH7057288 是一种选择性靶向 NTRK 阳性癌症的神经营养性酪氨酸受体激酶 (NTRK) 抑制剂。 NTRK融合是极具吸引力和有前途的治疗靶点,为了将CH7057288作为患者的新治疗选择,能够快速供应大量这种创新药物用于GLP毒理学研究和首次人体试验至关重要(FIH)研究。通过采用 11 步工艺,我们能够以高纯度和优异的收率从 2-(3-氨基苯基)-2-甲基丙酸甲酯合成 CH7057288。该过程的显着特征包括连续双环化,产生四元苯并呋喃稠合杂环。通过采用该工艺,我们成功生产了总计5.5公斤的CH7057288。
更新日期:2024-01-03
中文翻译:

CH7057288(一种含苯并呋喃的选择性 NTRK 抑制剂)的早期工艺开发
这项工作描述了 CH7057288 的可扩展制造工艺的开发,CH7057288 是一种选择性靶向 NTRK 阳性癌症的神经营养性酪氨酸受体激酶 (NTRK) 抑制剂。 NTRK融合是极具吸引力和有前途的治疗靶点,为了将CH7057288作为患者的新治疗选择,能够快速供应大量这种创新药物用于GLP毒理学研究和首次人体试验至关重要(FIH)研究。通过采用 11 步工艺,我们能够以高纯度和优异的收率从 2-(3-氨基苯基)-2-甲基丙酸甲酯合成 CH7057288。该过程的显着特征包括连续双环化,产生四元苯并呋喃稠合杂环。通过采用该工艺,我们成功生产了总计5.5公斤的CH7057288。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号