Med ( IF 12.8 ) Pub Date : 2024-01-04 , DOI: 10.1016/j.medj.2023.12.004 Long Mao 1 , Namir Shaabani 2 , Xiaoying Zhang 3 , Can Jin 1 , Wanhong Xu 3 , Christopher Argent 4 , Yulia Kushnareva 1 , Colin Powers 2 , Karen Stegman 2 , Jia Liu 1 , Hui Xie 2 , Changxu Xu 3 , Yimei Bao 3 , Lijun Xu 3 , Yuren Zhang 3 , Haigang Yang 3 , Shengdian Qian 3 , Yong Hu 3 , Jianping Shao 3 , Can Zhang 3 , Tingting Li 3 , Yi Li 3 , Na Liu 3 , Zhenhao Lin 3 , Shanbo Wang 3 , Chao Wang 3 , Wei Shen 3 , Yuanlong Lin 5 , Dan Shu 5 , Zhenhong Zhu 1 , Olivia Kotoi 1 , Lisa Kerwin 2 , Qing Han 6 , Ludmila Chumakova 1 , John Teijaro 7 , Mike Royal 2 , Mark Brunswick 2 , Robert Allen 2 , Henry Ji 2 , Hongzhou Lu 5 , Xiao Xu 1
|
Background
Oral antiviral drugs with improved antiviral potency and safety are needed to address current challenges in clinical practice for treatment of COVID-19, including the risks of rebound, drug-drug interactions, and emerging resistance.
Methods
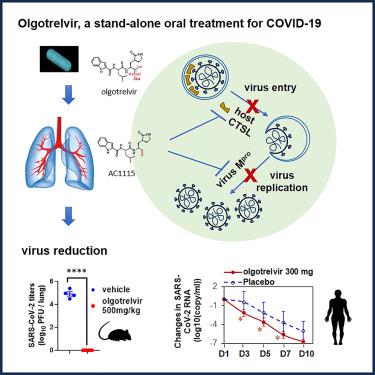
Olgotrelvir (STI-1558) is designed as a next-generation antiviral targeting the SARS-CoV-2 main protease (Mpro), an essential enzyme for SARS-CoV-2 replication, and human cathepsin L (CTSL), a key enzyme for SARS-CoV-2 entry into host cells.
Findings
Olgotrelvir is a highly bioavailable oral prodrug that is converted in plasma to its active form, AC1115. The dual mechanism of action of olgotrelvir and AC1115 was confirmed by enzyme activity inhibition assays and co-crystal structures of AC1115 with SARS-CoV-2 Mpro and human CTSL. AC1115 displayed antiviral activity by inhibiting replication of all tested SARS-CoV-2 variants in cell culture systems. Olgotrelvir also inhibited viral entry into cells using SARS-CoV-2 Spike-mediated pseudotypes by inhibition of host CTSL. In the K18-hACE2 transgenic mouse model of SARS-CoV-2-mediated disease, olgotrelvir significantly reduced the virus load in the lungs, prevented body weight loss, and reduced cytokine release and lung pathologies. Olgotrelvir demonstrated potent activity against the nirmatrelvir-resistant Mpro E166 mutants. Olgotrelvir showed enhanced oral bioavailability in animal models and in humans with significant plasma exposure without ritonavir. In phase I studies (ClinicalTrials.gov: NCT05364840 and NCT05523739), olgotrelvir demonstrated a favorable safety profile and antiviral activity.
Conclusions
Olgotrelvir is an oral inhibitor targeting Mpro and CTSL with high antiviral activity and plasma exposure and is a standalone treatment candidate for COVID-19.
Funding
Funded by Sorrento Therapeutics.
中文翻译:

Olgotrelvir 是 SARS-CoV-2 Mpro 和组织蛋白酶 L 的双重抑制剂,作为 COVID-19 的独立抗病毒口服干预候选药物
背景
需要具有更高抗病毒效力和安全性的口服抗病毒药物来应对当前治疗 COVID-19 临床实践中的挑战,包括反弹风险、药物相互作用和新出现的耐药性。
方法
Olgotrelvir (STI-1558) 被设计为下一代抗病毒药物,针对 SARS-CoV-2 主蛋白酶 (M pro )(SARS-CoV-2 复制的必需酶)和人组织蛋白酶 L (CTSL)(一种关键酶) SARS-CoV-2 进入宿主细胞。
发现
Olgotrelvir 是一种高生物利用度的口服前药,可在血浆中转化为其活性形式 AC1115。 olgotrelvir 和 AC1115 的双重作用机制通过酶活性抑制测定和 AC1115 与 SARS-CoV-2 M pro和人 CTSL 的共晶结构得到证实。 AC1115 通过抑制细胞培养系统中所有测试的 SARS-CoV-2 变体的复制来表现出抗病毒活性。 Olgotrelvir 还通过抑制宿主 CTSL 来抑制病毒利用 SARS-CoV-2 Spike 介导的假型病毒进入细胞。在 SARS-CoV-2 介导的疾病的 K18-hACE2 转基因小鼠模型中,olgotrelvir 显着降低了肺部的病毒载量,防止体重减轻,并减少细胞因子释放和肺部病理。 Olgotrelvir 对 nirmatrelvir 耐药的 M pro E166 突变体表现出有效的活性。在没有利托那韦的情况下,在动物模型和具有显着血浆暴露的人类中,Olgotrelvir 显示出增强的口服生物利用度。在 I 期研究(ClinicalTrials.gov:NCT05364840 和 NCT05523739)中,olgotrelvir 表现出良好的安全性和抗病毒活性。
结论
Olgotrelvir 是一种针对 M pro和 CTSL 的口服抑制剂,具有高抗病毒活性和血浆暴露量,是 COVID-19 的独立治疗候选药物。
资金
由索伦托疗法资助。






























 京公网安备 11010802027423号
京公网安备 11010802027423号