Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adjusting Li+ Solvation Structures via Dipole–Dipole Interaction to Construct Inorganic-Rich Interphase for High-Performance Li Metal Batteries
Small ( IF 13.0 ) Pub Date : 2024-01-02 , DOI: 10.1002/smll.202308995 Chuan Wang 1 , Sheng Liu 1 , Haoyang Xu 1 , Xinxiang Wang 1 , Guilei Tian 1 , Fengxia Fan 1 , Pengfei Liu 1 , Shuhan Wang 1 , Chenrui Zeng 1 , Chaozhu Shu 1
Small ( IF 13.0 ) Pub Date : 2024-01-02 , DOI: 10.1002/smll.202308995 Chuan Wang 1 , Sheng Liu 1 , Haoyang Xu 1 , Xinxiang Wang 1 , Guilei Tian 1 , Fengxia Fan 1 , Pengfei Liu 1 , Shuhan Wang 1 , Chenrui Zeng 1 , Chaozhu Shu 1
Affiliation
|
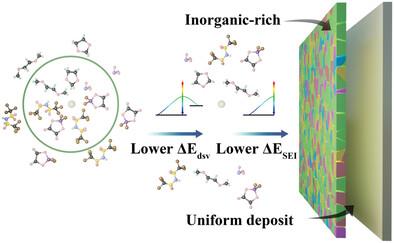
Practical applications of lithium metal batteries are limited by unstable solid electrolyte interphase (SEI) and uncontrollable dendrite Li deposition. Regulating the solvation structure of Li+ via modifying electrolyte components enables optimizing the structure of the SEI and realizing dendrite-free Li deposition. In this work, it is found that the ionic–dipole interactions between the electron-deficient B atoms in lithium oxalyldifluoro borate (LiDFOB) and the O atoms in the DME solvent molecule can weaken the interaction between the DME molecule and Li+, accelerating the desolvation of Li+. On this basis, the ionic–dipole interactions facilitate the entry of abundant anions into the inner solvation sheath of Li+, which promotes the formation of inorganic-rich SEI. In addition, the interaction between DFOB− and DME molecules reduces the highest occupied molecular orbital energy level of DME molecules in electrolytes, which improves the oxidative stability of the electrolytes system. As a result, the Li||Li cells in LiDFOB-containing electrolytes exhibit an excellent cyclability of over 1800 h with a low overpotential of 18.2 mV, and the Li||LiFePO4 full cells display a high-capacity retention of 93.4% after 100 cycles with a high Coulombic efficiency of 99.3%.
中文翻译:

通过偶极-偶极相互作用调整Li+溶剂化结构构建高性能锂金属电池的富无机界面相
锂金属电池的实际应用受到不稳定的固体电解质界面(SEI)和不可控的枝晶锂沉积的限制。通过改变电解质成分来调节Li +的溶剂化结构,可以优化SEI的结构并实现无枝晶的锂沉积。本工作发现草酰基二氟硼酸锂(LiDFOB)中的缺电子B原子与DME溶剂分子中的O原子之间的离子偶极相互作用可以减弱DME分子与Li +之间的相互作用,加速Li +的去溶剂化。在此基础上,离子-偶极相互作用促进丰富的阴离子进入Li +的内部溶剂化鞘,从而促进富含无机物的SEI的形成。此外,DFOB -与DME分子之间的相互作用降低了电解质中DME分子的最高占据分子轨道能级,从而提高了电解质体系的氧化稳定性。结果,含LiDFOB电解质中的Li||Li电池表现出超过1800小时的优异循环性能以及18.2 mV的低过电位,并且Li||LiFePO 4全电池在使用后表现出93.4%的高容量保持率。 100 次循环,库仑效率高达 99.3%。
更新日期:2024-01-02
中文翻译:

通过偶极-偶极相互作用调整Li+溶剂化结构构建高性能锂金属电池的富无机界面相
锂金属电池的实际应用受到不稳定的固体电解质界面(SEI)和不可控的枝晶锂沉积的限制。通过改变电解质成分来调节Li +的溶剂化结构,可以优化SEI的结构并实现无枝晶的锂沉积。本工作发现草酰基二氟硼酸锂(LiDFOB)中的缺电子B原子与DME溶剂分子中的O原子之间的离子偶极相互作用可以减弱DME分子与Li +之间的相互作用,加速Li +的去溶剂化。在此基础上,离子-偶极相互作用促进丰富的阴离子进入Li +的内部溶剂化鞘,从而促进富含无机物的SEI的形成。此外,DFOB -与DME分子之间的相互作用降低了电解质中DME分子的最高占据分子轨道能级,从而提高了电解质体系的氧化稳定性。结果,含LiDFOB电解质中的Li||Li电池表现出超过1800小时的优异循环性能以及18.2 mV的低过电位,并且Li||LiFePO 4全电池在使用后表现出93.4%的高容量保持率。 100 次循环,库仑效率高达 99.3%。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号