当前位置:
X-MOL 学术
›
J. Environ. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Di-imine Schiff base inhibitor for carbon steel corrosion in 1 M HCl: Electrochemical, surface and theoretical investigations
Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2024-01-03 , DOI: 10.1016/j.jece.2023.111861 A. Elaraby , Khaled Faisal Qasim , Shaimaa K. Mohamed , E.A. El-Sharkawy , Samar Abdelhamed
Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2024-01-03 , DOI: 10.1016/j.jece.2023.111861 A. Elaraby , Khaled Faisal Qasim , Shaimaa K. Mohamed , E.A. El-Sharkawy , Samar Abdelhamed
|
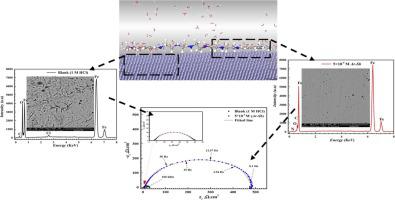
Many strategies for an efficient inhibitor preparing were proposed for metals protection against corrosion in last decades. Herein, the adsorption and inhibitory effect of aromatic di-imine Schiff base inhibitor () namely (N1-(2-(((E)− 4-(dimethylamino)benzylidene)amino)ethyl)-N2-(2-((2-((2(Z)− 4-dimethylamino)ben- zylidene)amino) ethyl) amino) ethyl) amino) ethyl)ethane-1,2-diamine) on carbon steel () in 1 M HCl solution was investigated experimentally and theoretically. The electrochemical measurements (EMs) such as electrochemical impedance spectroscopy (EIS), Potentiodynamic polarization (PDP) and cyclic voltammetry (CV) techniques exhibited the anticorrosion performance of . Also, the adsorption of on surface at different temperatures and immersion times was studied using PDP and EIS which indicated formation of a protective film layer of responsible for corrosion mitigation. inhibitor provided 95.79% inhibition efficiency at 5 × 10 M which reflected blocking of active sites through the adsorption process which was studied using various adsorption isotherms. surface morphology was studied using scanning electron microscopy (SEM), energy dispersive X-ray (EDX) and atomic force microscope (AFM) reflecting the inhibition role of in protection. The data obtained from PDP suggested that inhibitor acted as mixed-type inhibitor. Also, quantum calculations such as density functional theory (DFT) and Monte Carlo (MCs) verified the inhibitive performance of for surface with the experimental results.
中文翻译:

用于 1 M HCl 中碳钢腐蚀的二亚胺席夫碱抑制剂:电化学、表面和理论研究
在过去的几十年里,人们提出了许多制备有效抑制剂的策略来保护金属免受腐蚀。这里芳香族二亚胺席夫碱抑制剂()的吸附抑制作用即(N1-(2-(((E)− 4-(二甲氨基)亚苯亚甲基)氨基)乙基)-N2-(2-((2通过实验研究了 1 M HCl 溶液中碳钢 () 上的 -((2(Z)− 4-二甲基氨基)亚苯亚基)氨基)乙基)氨基)乙基)氨基)乙基)乙烷-1,2-二胺)理论上。电化学测量(EM),如电化学阻抗谱(EIS)、动电位极化(PDP)和循环伏安法(CV)技术显示了其防腐性能。此外,使用 PDP 和 EIS 研究了不同温度和浸泡时间下表面的吸附情况,表明形成了负责缓解腐蚀的保护膜层。抑制剂在 5 × 10 M 处提供 95.79% 的抑制效率,这反映了通过使用各种吸附等温线研究的吸附过程对活性位点的阻断。使用扫描电子显微镜(SEM)、能量色散X射线(EDX)和原子力显微镜(AFM)研究表面形貌,反映其在保护中的抑制作用。从 PDP 获得的数据表明该抑制剂充当混合型抑制剂。此外,密度泛函理论(DFT)和蒙特卡罗(MCs)等量子计算通过实验结果验证了表面的抑制性能。
更新日期:2024-01-03
中文翻译:

用于 1 M HCl 中碳钢腐蚀的二亚胺席夫碱抑制剂:电化学、表面和理论研究
在过去的几十年里,人们提出了许多制备有效抑制剂的策略来保护金属免受腐蚀。这里芳香族二亚胺席夫碱抑制剂()的吸附抑制作用即(N1-(2-(((E)− 4-(二甲氨基)亚苯亚甲基)氨基)乙基)-N2-(2-((2通过实验研究了 1 M HCl 溶液中碳钢 () 上的 -((2(Z)− 4-二甲基氨基)亚苯亚基)氨基)乙基)氨基)乙基)氨基)乙基)乙烷-1,2-二胺)理论上。电化学测量(EM),如电化学阻抗谱(EIS)、动电位极化(PDP)和循环伏安法(CV)技术显示了其防腐性能。此外,使用 PDP 和 EIS 研究了不同温度和浸泡时间下表面的吸附情况,表明形成了负责缓解腐蚀的保护膜层。抑制剂在 5 × 10 M 处提供 95.79% 的抑制效率,这反映了通过使用各种吸附等温线研究的吸附过程对活性位点的阻断。使用扫描电子显微镜(SEM)、能量色散X射线(EDX)和原子力显微镜(AFM)研究表面形貌,反映其在保护中的抑制作用。从 PDP 获得的数据表明该抑制剂充当混合型抑制剂。此外,密度泛函理论(DFT)和蒙特卡罗(MCs)等量子计算通过实验结果验证了表面的抑制性能。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号