当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced N2 Adsorption and Activation by Combining Re Clusters and In Vacancies as Dual Sites for Efficient and Selective Electrochemical NH3 Synthesis
Nano Letters ( IF 9.6 ) Pub Date : 2024-01-03 , DOI: 10.1021/acs.nanolett.3c04416
Shaoquan Li 1, 2 , Yi-Tao Liu 3 , Yong-Chao Zhang 1 , Yue Du 1 , Jian Gao 1 , Jingru Zhai 4 , Yue Liang 1 , Caidi Han 1 , Xiao-Dong Zhu 1
Nano Letters ( IF 9.6 ) Pub Date : 2024-01-03 , DOI: 10.1021/acs.nanolett.3c04416
Shaoquan Li 1, 2 , Yi-Tao Liu 3 , Yong-Chao Zhang 1 , Yue Du 1 , Jian Gao 1 , Jingru Zhai 4 , Yue Liang 1 , Caidi Han 1 , Xiao-Dong Zhu 1
Affiliation
|
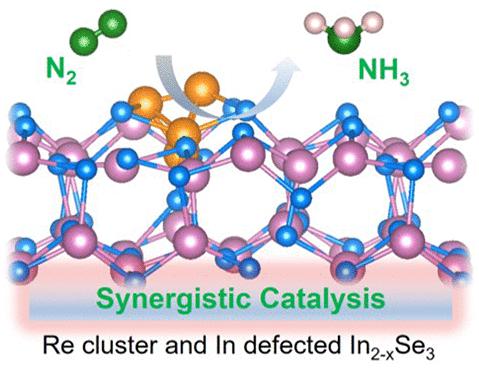
The electrochemical N2 reduction reaction (NRR) is a green and energy-saving sustainable technology for NH3 production. However, high activity and high selectivity can hardly be achieved in the same catalyst, which severely restricts the development of the electrochemical NRR. In2Se3 with partially occupied p-orbitals can suppress the H2 evolution reaction (HER), which shows excellent selectivity in the electrochemical NRR. The presence of VIn can simultaneously provide active sites and confine Re clusters through strong charge transfer. Additionally, well-isolated Re clusters stabilized on In2Se3 by the confinement effect of VIn result in Re-VIn active sites with maximum availability. By combining Re clusters and VIn as dual sites for spontaneous N2 adsorption and activation, the electrochemical NRR performance is enhanced significantly. As a result, the Re-In2Se3-VIn/CC catalyst delivers a high NH3 yield rate (26.63 μg h–1 cm–2) and high FEs (30.8%) at −0.5 V vs RHE.
中文翻译:

通过结合 Re 团簇和空位作为双位点增强 N2 吸附和活化,实现高效、选择性电化学 NH3 合成
电化学N 2还原反应(NRR)是一种绿色、节能、可持续的NH 3生产技术。然而,在同一催化剂中很难同时实现高活性和高选择性,这严重限制了电化学NRR的发展。在2 Se 3中,部分占据的p轨道可以抑制H 2析出反应(HER),这在电化学NRR中表现出优异的选择性。 V In的存在可以同时提供活性位点并通过强电荷转移限制 Re 团簇。此外,通过 V In的限制效应,隔离良好的 Re 团簇稳定在 In 2 Se 3上,导致 Re-V In活性位点具有最大的可用性。通过将Re簇和V In结合作为自发N 2吸附和活化的双位点,电化学NRR性能显着增强。因此,Re-In 2 Se 3 -V In /CC 催化剂在 -0.5 V vs RHE 条件下具有高 NH 3收率(26.63 μg h –1 cm –2 )和高 FE(30.8%)。
更新日期:2024-01-03
中文翻译:

通过结合 Re 团簇和空位作为双位点增强 N2 吸附和活化,实现高效、选择性电化学 NH3 合成
电化学N 2还原反应(NRR)是一种绿色、节能、可持续的NH 3生产技术。然而,在同一催化剂中很难同时实现高活性和高选择性,这严重限制了电化学NRR的发展。在2 Se 3中,部分占据的p轨道可以抑制H 2析出反应(HER),这在电化学NRR中表现出优异的选择性。 V In的存在可以同时提供活性位点并通过强电荷转移限制 Re 团簇。此外,通过 V In的限制效应,隔离良好的 Re 团簇稳定在 In 2 Se 3上,导致 Re-V In活性位点具有最大的可用性。通过将Re簇和V In结合作为自发N 2吸附和活化的双位点,电化学NRR性能显着增强。因此,Re-In 2 Se 3 -V In /CC 催化剂在 -0.5 V vs RHE 条件下具有高 NH 3收率(26.63 μg h –1 cm –2 )和高 FE(30.8%)。

































 京公网安备 11010802027423号
京公网安备 11010802027423号