当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
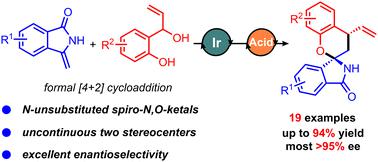
Enantioselective synthesis of spiro-N,O-ketals via iridium and Brønsted acid co-catalyzed asymmetric formal [4+2] cycloaddition
Chemical Communications ( IF 4.3 ) Pub Date : 2024-01-03 , DOI: 10.1039/d3cc05923e Xiang-Qi Xie 1 , Xingguang Li 1 , Pei-Nian Liu 1
Chemical Communications ( IF 4.3 ) Pub Date : 2024-01-03 , DOI: 10.1039/d3cc05923e Xiang-Qi Xie 1 , Xingguang Li 1 , Pei-Nian Liu 1
Affiliation
|
We present an iridium and Brønsted acid co-catalyzed enantioselective formal [4+2] cycloaddition reaction of cyclic enamides with 2-(1-hydroxyallyl)phenols. This method yields a wide range of N-unsubstituted spiro-N,O-ketals, with good efficiency (up to 94%) and excellent enantioselectivities (most >95% ee). The protocol features easy scale-up and facile product derivatization.
中文翻译:

铱和布朗斯台德酸共催化不对称形式[4+2]环加成对映选择性合成螺-N,O-缩酮
我们提出了铱和布朗斯台德酸共催化环状烯酰胺与 2-(1-羟基烯丙基)苯酚的对映选择性形式 [4+2] 环加成反应。该方法可产生多种N-未取代的螺-N , O-缩酮,具有良好的效率(高达 94%)和优异的对映选择性(大多数 >95% ee)。该方案具有易于放大和产品衍生化的特点。
更新日期:2024-01-03
中文翻译:

铱和布朗斯台德酸共催化不对称形式[4+2]环加成对映选择性合成螺-N,O-缩酮
我们提出了铱和布朗斯台德酸共催化环状烯酰胺与 2-(1-羟基烯丙基)苯酚的对映选择性形式 [4+2] 环加成反应。该方法可产生多种N-未取代的螺-N , O-缩酮,具有良好的效率(高达 94%)和优异的对映选择性(大多数 >95% ee)。该方案具有易于放大和产品衍生化的特点。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号