Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
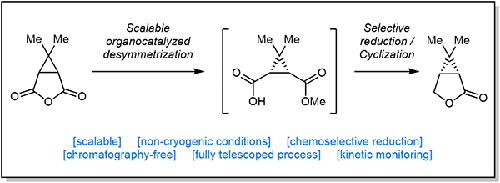
Efficient and Scalable Synthesis of 6,6-Dimethyl-3-oxabicyclo [3.1.0]hexan-2-one through Organocatalyzed Desymmetrization and Chemoselective Reduction
Synthesis ( IF 2.2 ) Pub Date : 2024-01-02 , DOI: 10.1055/a-2217-0996 Guillaume Tintori 1 , Clément Jacob 1 , Christine Delsarte 1 , François Potié 1 , Laurence Grimaud 2 , maxime Vitale 3 , Pierre-Georges Echeverria 1
中文翻译:

通过有机催化去对称和化学选择性还原高效、可规模化合成 6,6-二甲基-3-氧杂双环 [3.1.0]己-2-酮
更新日期:2024-01-03
Synthesis ( IF 2.2 ) Pub Date : 2024-01-02 , DOI: 10.1055/a-2217-0996 Guillaume Tintori 1 , Clément Jacob 1 , Christine Delsarte 1 , François Potié 1 , Laurence Grimaud 2 , maxime Vitale 3 , Pierre-Georges Echeverria 1
Affiliation
|
The development of a robust process to 6,6-dimethyl-3-oxabicyclo[3.1.0]hexan-2-one, a key intermediate toward several active compounds of interest, is described. A scalable organocatalyzed desymmetrization was first studied and the reaction proved to be compatible with standard dilution. A kinetic study was performed to elucidate substrate and catalyst concentration dependences. Next, a chemoselective reduction of the carboxylic acid was developed. The scalability was demonstrated on 100 g scale through a fully telescoped process.
中文翻译:

通过有机催化去对称和化学选择性还原高效、可规模化合成 6,6-二甲基-3-氧杂双环 [3.1.0]己-2-酮
描述了 6,6-二甲基-3-氧杂双环[3.1.0]己-2-酮(几种感兴趣的活性化合物的关键中间体)的稳健工艺的开发。首先研究了可扩展的有机催化去对称反应,并证明该反应与标准稀释相容。进行动力学研究以阐明底物和催化剂浓度依赖性。接下来,开发了羧酸的化学选择性还原。通过完全伸缩过程在 100 g 规模上展示了可扩展性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号