当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergy of Single-Atom Fe1 and Ce–Ov Sites on Mesoporous CeO2–Al2O3 for Efficient Selective Catalytic Reduction of NO with CO
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-01-03 , DOI: 10.1021/acscatal.3c04682
Yuting Bai 1 , Xupeng Zong 1 , Chengwen Jin 1 , Shudong Wang 1 , Sheng Wang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-01-03 , DOI: 10.1021/acscatal.3c04682
Yuting Bai 1 , Xupeng Zong 1 , Chengwen Jin 1 , Shudong Wang 1 , Sheng Wang 1
Affiliation
|
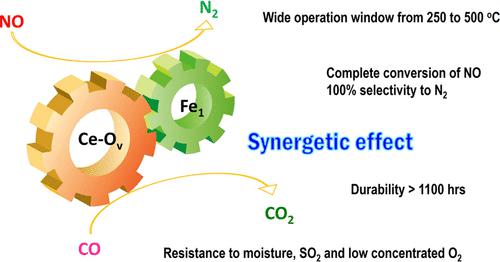
Nonprecious transition-metal oxides, especially Fe-, Cu-, Co-, and Mn-containing mixed oxides, have been regarded as promising alternatives for noble metal catalysts for the abatement of NOx contamination. However, the identification of the real catalytically active sites for these mixed oxides remains unclear in most cases, which limits our in-depth understanding of the intrinsic mechanism. Here, we comprehensively investigated an iron–cerium–aluminum oxide (Fe1/CeO2–Al2O3) prepared with a co-precipitation method. Structural identification confirmed that Fe sites are atomically dispersed, bonding with four O atoms in the first coordination shell and with two Ce atoms in the second shell on average. Highly efficient removal of NO with 100% selectivity toward N2 has been achieved over these sites at a temperature as low as 250 °C. In situ characterizations and computational studies revealed that the high activity and N2 selectivity of Fe1/CeO2–Al2O3 can be attributed to the synergetic effect of the single-atomic Fe1 site and surrounding Ce–Ov, which intensively promotes the adsorption of NO molecules and N2O intermediates. Subsequently, Ce–Ov facilitates the N–O dissociation toward N2 and then is regenerated with CO, forming CO2 as a product. The present results provide valuable insights into the mechanism of transition-metal oxide catalysts for the NO–CO reaction and offer useful guidance for designing catalysts with high activity and selectivity.
中文翻译:

介孔 CeO2-Al2O3 上单原子 Fe1 和 Ce-Ov 位点的协同作用,用于 CO 高效选择性催化还原 NO
非贵金属过渡金属氧化物,特别是含Fe、Cu、Co和Mn的混合氧化物,已被认为是用于减少NO x污染的贵金属催化剂的有前途的替代品。然而,在大多数情况下,这些混合氧化物真正的催化活性位点的识别仍不清楚,这限制了我们对其内在机制的深入理解。在这里,我们全面研究了用共沉淀法制备的铁-铈-铝氧化物(Fe 1 /CeO 2 -Al 2 O 3 )。结构鉴定证实Fe位点是原子分散的,平均与第一配位层中的四个O原子成键,并与第二个配位层中的两个Ce原子成键。在低至 250 °C 的温度下,这些位点已经实现了对 N 2具有 100% 选择性的高效去除 NO 。原位表征和计算研究表明,Fe 1 /CeO 2 –Al 2 O 3的高活性和 N 2选择性可归因于单原子 Fe 1位点和周围 Ce-O v的协同效应,这强烈促进NO分子和N 2 O中间体的吸附。随后,Ce-O v促进N-O解离成N 2,然后用CO再生,形成CO 2作为产物。目前的结果为了解过渡金属氧化物催化剂用于NO-CO反应的机理提供了有价值的见解,并为设计具有高活性和选择性的催化剂提供了有用的指导。
更新日期:2024-01-03
中文翻译:

介孔 CeO2-Al2O3 上单原子 Fe1 和 Ce-Ov 位点的协同作用,用于 CO 高效选择性催化还原 NO
非贵金属过渡金属氧化物,特别是含Fe、Cu、Co和Mn的混合氧化物,已被认为是用于减少NO x污染的贵金属催化剂的有前途的替代品。然而,在大多数情况下,这些混合氧化物真正的催化活性位点的识别仍不清楚,这限制了我们对其内在机制的深入理解。在这里,我们全面研究了用共沉淀法制备的铁-铈-铝氧化物(Fe 1 /CeO 2 -Al 2 O 3 )。结构鉴定证实Fe位点是原子分散的,平均与第一配位层中的四个O原子成键,并与第二个配位层中的两个Ce原子成键。在低至 250 °C 的温度下,这些位点已经实现了对 N 2具有 100% 选择性的高效去除 NO 。原位表征和计算研究表明,Fe 1 /CeO 2 –Al 2 O 3的高活性和 N 2选择性可归因于单原子 Fe 1位点和周围 Ce-O v的协同效应,这强烈促进NO分子和N 2 O中间体的吸附。随后,Ce-O v促进N-O解离成N 2,然后用CO再生,形成CO 2作为产物。目前的结果为了解过渡金属氧化物催化剂用于NO-CO反应的机理提供了有价值的见解,并为设计具有高活性和选择性的催化剂提供了有用的指导。

































 京公网安备 11010802027423号
京公网安备 11010802027423号