当前位置:
X-MOL 学术
›
Appl. Catal. B Environ. Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insight into hydroxyl groups in anchoring Ir single–atoms on vacancy–deficient rutile TiO2 supports for selective catalytic oxidation of ammonia
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2024-01-02 , DOI: 10.1016/j.apcatb.2023.123684 Wenqing Xu , Yixi Wang , Hong He , Jun Yang , Yang Yang , Jinzhu Ma , Chaoqun Li , Tingyu Zhu
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2024-01-02 , DOI: 10.1016/j.apcatb.2023.123684 Wenqing Xu , Yixi Wang , Hong He , Jun Yang , Yang Yang , Jinzhu Ma , Chaoqun Li , Tingyu Zhu
|
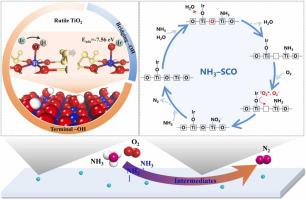
High–performance catalysts are extremely required for controlling NH emission via selective catalytic oxidation (SCO), and the anchoring structural feature of active sites is a key prerequisite for developing them. This study confirms the importance of hydroxyl groups on vacancy–deficient reducible oxides as active groups. On the one hand, spontaneous atomic dispersion of active metal Ir is promoted by the abundant terminal hydroxyl groups. On the other hand, Ir cations anchor on the TiO surface through exchange with H in Ti–OH groups, and thus occupy the Brönsted acid sites. The adsorption strength of NH is another key factor affecting the reaction rate–determining step, namely NH dehydrogenation, which occurs at a faster rate in the coordinated L–NH rather than the ionic B–NH. Meanwhile, the coordinated L–NH significantly avoids the competitive adsorption of water vapor in the NH–SCO reaction by reducing the number of hydrogen bonding. The TOF of preferred 0.8Ir/TiO sample is significantly higher than 0.2Ir/TiO sample, although Ir is almost always atomic dispersed. Finally, NH conversion is 85% in a wet circumstance (5% HO) at 240 °C (GHSV = 85 000 h), with a N selectivity of up to 65% on 0.8Ir/TiO sample.
中文翻译:

深入研究羟基在缺位金红石 TiO2 载体上锚定 Ir 单原子的作用,用于氨的选择性催化氧化
通过选择性催化氧化(SCO)控制NH排放非常需要高性能催化剂,而活性位点的锚定结构特征是开发它们的关键先决条件。这项研究证实了羟基作为活性基团对缺位可还原氧化物的重要性。一方面,丰富的末端羟基促进了活性金属Ir的自发原子分散。另一方面,Ir 阳离子通过与 Ti-OH 基团中的 H 交换而锚定在 TiO2 表面,从而占据布朗斯台德酸位。 NH 的吸附强度是影响反应速率决定步骤(即 NH 脱氢)的另一个关键因素,其在配位的 L-NH 中发生的速率比离子 B-NH 中更快。同时,配位的L-NH通过减少氢键数量,显着避免了NH-SCO反应中水蒸气的竞争吸附。优选的0.8Ir/TiO样品的TOF显着高于0.2Ir/TiO样品,尽管Ir几乎总是原子分散的。最后,在 240 °C(GHSV = 85 000 h)的潮湿环境(5% H2O)下,NH 转化率为 85%,0.8Ir/TiO 样品上的 N 选择性高达 65%。
更新日期:2024-01-02
中文翻译:

深入研究羟基在缺位金红石 TiO2 载体上锚定 Ir 单原子的作用,用于氨的选择性催化氧化
通过选择性催化氧化(SCO)控制NH排放非常需要高性能催化剂,而活性位点的锚定结构特征是开发它们的关键先决条件。这项研究证实了羟基作为活性基团对缺位可还原氧化物的重要性。一方面,丰富的末端羟基促进了活性金属Ir的自发原子分散。另一方面,Ir 阳离子通过与 Ti-OH 基团中的 H 交换而锚定在 TiO2 表面,从而占据布朗斯台德酸位。 NH 的吸附强度是影响反应速率决定步骤(即 NH 脱氢)的另一个关键因素,其在配位的 L-NH 中发生的速率比离子 B-NH 中更快。同时,配位的L-NH通过减少氢键数量,显着避免了NH-SCO反应中水蒸气的竞争吸附。优选的0.8Ir/TiO样品的TOF显着高于0.2Ir/TiO样品,尽管Ir几乎总是原子分散的。最后,在 240 °C(GHSV = 85 000 h)的潮湿环境(5% H2O)下,NH 转化率为 85%,0.8Ir/TiO 样品上的 N 选择性高达 65%。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号