当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enzymatic Assembly of Diverse Lactone Structures: An Intramolecular C–H Functionalization Strategy
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-01-02 , DOI: 10.1021/jacs.3c11722 Daniel J Wackelin 1 , Runze Mao 1 , Kathleen M Sicinski 1 , Yutao Zhao 1 , Anuvab Das 1 , Kai Chen 1 , Frances H Arnold 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-01-02 , DOI: 10.1021/jacs.3c11722 Daniel J Wackelin 1 , Runze Mao 1 , Kathleen M Sicinski 1 , Yutao Zhao 1 , Anuvab Das 1 , Kai Chen 1 , Frances H Arnold 1
Affiliation
|
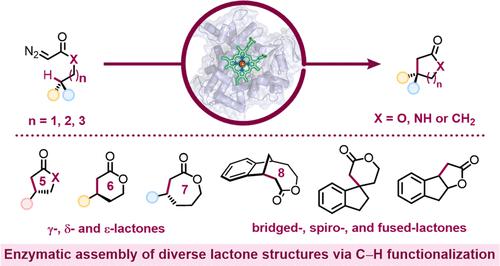
Lactones are cyclic esters with extensive applications in materials science, medicinal chemistry, and the food and perfume industries. Nature’s strategy for the synthesis of many lactones found in natural products always relies on a single type of retrosynthetic strategy, a C–O bond disconnection. Here, we describe a set of laboratory-engineered enzymes that use a new-to-nature C–C bond-forming strategy to assemble diverse lactone structures. These engineered “carbene transferases” catalyze intramolecular carbene insertions into benzylic or allylic C–H bonds, which allow for the synthesis of lactones with different ring sizes and ring scaffolds from simple starting materials. Starting from a serine-ligated cytochrome P450 variant previously engineered for other carbene-transfer activities, directed evolution generated a variant P411-LAS-5247, which exhibits a high activity for constructing a five-membered ε-lactone, lactam, and cyclic ketone products (up to 5600 total turnovers (TTN) and >99% enantiomeric excess (ee)). Further engineering led to variants P411-LAS-5249 and P411-LAS-5264, which deliver six-membered δ-lactones and seven-membered ε-lactones, respectively, overcoming the thermodynamically unfavorable ring strain associated with these products compared to the γ-lactones. This new carbene-transfer activity was further extended to the synthesis of complex lactone scaffolds based on fused, bridged, and spiro rings. The enzymatic platform developed here complements natural biosynthetic strategies for lactone assembly and expands the structural diversity of lactones accessible through C–H functionalization.
中文翻译:

多种内酯结构的酶促组装:分子内 C-H 官能化策略
内酯是环酯,在材料科学、药物化学以及食品和香水工业中有着广泛的应用。自然界中天然产物中发现的许多内酯的合成策略始终依赖于单一类型的逆合成策略,即 C-O 键断开。在这里,我们描述了一组实验室工程酶,它们使用全新的 C-C 键形成策略来组装不同的内酯结构。这些工程化的“卡宾转移酶”催化分子内卡宾插入苄基或烯丙基C-H键,从而可以从简单的起始材料合成具有不同环尺寸和环支架的内酯。从先前设计用于其他卡宾转移活性的丝氨酸连接细胞色素 P450 变体开始,定向进化产生了变体 P411-LAS-5247,该变体在构建五元 ε-内酯、内酰胺和环酮产物方面表现出高活性(高达 5600 总营业额 (TTN) 和 >99% 对映体过量 (ee))。进一步的工程设计产生了变体 P411-LAS-5249 和 P411-LAS-5264,它们分别提供六元 δ-内酯和七元 ε-内酯,克服了与 γ- 相比,与这些产品相关的热力学不利环应变。内酯。这种新的卡宾转移活性进一步扩展到基于稠合环、桥环和螺环的复杂内酯支架的合成。这里开发的酶平台补充了内酯组装的天然生物合成策略,并通过 C-H 官能化扩展了内酯的结构多样性。
更新日期:2024-01-02
中文翻译:

多种内酯结构的酶促组装:分子内 C-H 官能化策略
内酯是环酯,在材料科学、药物化学以及食品和香水工业中有着广泛的应用。自然界中天然产物中发现的许多内酯的合成策略始终依赖于单一类型的逆合成策略,即 C-O 键断开。在这里,我们描述了一组实验室工程酶,它们使用全新的 C-C 键形成策略来组装不同的内酯结构。这些工程化的“卡宾转移酶”催化分子内卡宾插入苄基或烯丙基C-H键,从而可以从简单的起始材料合成具有不同环尺寸和环支架的内酯。从先前设计用于其他卡宾转移活性的丝氨酸连接细胞色素 P450 变体开始,定向进化产生了变体 P411-LAS-5247,该变体在构建五元 ε-内酯、内酰胺和环酮产物方面表现出高活性(高达 5600 总营业额 (TTN) 和 >99% 对映体过量 (ee))。进一步的工程设计产生了变体 P411-LAS-5249 和 P411-LAS-5264,它们分别提供六元 δ-内酯和七元 ε-内酯,克服了与 γ- 相比,与这些产品相关的热力学不利环应变。内酯。这种新的卡宾转移活性进一步扩展到基于稠合环、桥环和螺环的复杂内酯支架的合成。这里开发的酶平台补充了内酯组装的天然生物合成策略,并通过 C-H 官能化扩展了内酯的结构多样性。































 京公网安备 11010802027423号
京公网安备 11010802027423号