当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An electrochemical access to 2-amino-2,3-dihydro-1,4-benzodioxanes derived from hydroxytyrosol
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2024-01-02 , DOI: 10.1039/d3ob01858j Anne Neudorffer 1 , Patrick Deschamps 1 , Martine Largeron 1 , Brigitte Deguin 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2024-01-02 , DOI: 10.1039/d3ob01858j Anne Neudorffer 1 , Patrick Deschamps 1 , Martine Largeron 1 , Brigitte Deguin 1
Affiliation
|
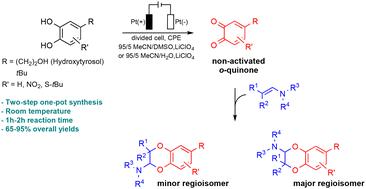
The anodic oxidation of a natural antioxidative catechol, hydroxytyrosol, was developed in an acetonitrile/dimethylsulfoxide (or acetonitrile/water) solvent mixture to produce in a stable way the resulting non-activated o-quinone and generate structural analogues. 2-Amino-2,3-dihydro-1,4-benzodioxane derivatives were obtained as two regioisomers in good to high overall yields (65–90%) and 1 : 3 ratios, through an inverse electron demand Diels–Alder (IEDDA) reaction between the electrogenerated o-quinone and tertiary enamines. The insertion of an electron withdrawing (or electron donating) group on the catechol modified their relative proportions, so that the reaction became regiospecific. With some aliphatic enamines, a competitive 1,6-Michael addition took place, affording 2-hydroxy-1,2,4,5-tetrahydrobenzo[d]oxepine compounds.
中文翻译:

电化学制备源自羟基酪醇的 2-氨基-2,3-二氢-1,4-苯并二恶烷
天然抗氧化儿茶酚羟基酪醇的阳极氧化是在乙腈/二甲亚砜(或乙腈/水)溶剂混合物中开发的,以稳定的方式产生所得的非活化邻醌并产生结构类似物。通过逆电子需求 Diels-Alder (IEDDA),以良好到高的总产率 (65–90%) 和 1:3 的比例获得了两种区域异构体形式的 2-氨基-2,3-二氢-1,4-苯并二恶烷衍生物电生成的邻醌和叔烯胺之间的反应。在儿茶酚上插入吸电子(或供电子)基团改变了它们的相对比例,从而使反应变得区域特异性。与一些脂肪族烯胺发生竞争性1,6-迈克尔加成,得到2-羟基-1,2,4,5-四氢苯并[ d ]氧杂环己烷化合物。
更新日期:2024-01-02
中文翻译:

电化学制备源自羟基酪醇的 2-氨基-2,3-二氢-1,4-苯并二恶烷
天然抗氧化儿茶酚羟基酪醇的阳极氧化是在乙腈/二甲亚砜(或乙腈/水)溶剂混合物中开发的,以稳定的方式产生所得的非活化邻醌并产生结构类似物。通过逆电子需求 Diels-Alder (IEDDA),以良好到高的总产率 (65–90%) 和 1:3 的比例获得了两种区域异构体形式的 2-氨基-2,3-二氢-1,4-苯并二恶烷衍生物电生成的邻醌和叔烯胺之间的反应。在儿茶酚上插入吸电子(或供电子)基团改变了它们的相对比例,从而使反应变得区域特异性。与一些脂肪族烯胺发生竞争性1,6-迈克尔加成,得到2-羟基-1,2,4,5-四氢苯并[ d ]氧杂环己烷化合物。































 京公网安备 11010802027423号
京公网安备 11010802027423号