当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evolution of the Synthetic Process to an Advanced GPR40 Agonist
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-01-02 , DOI: 10.1021/acs.oprd.3c00433 Xiaoping Hou 1 , Lucas W. Hernandez 2 , Elizabeth A. Jurica 1 , Rulin Zhao 1 , Bei Wang 1 , Michael Wong 1 , Jung-Hui Sun 1 , Dawn Sun 1 , Dauh-Rurng Wu 1 , Changxia Yuan 2 , Michael Hay 2 , Miao Yu 2 , Ximao Wu 1 , Yanting Huang 1 , Bruce A. Ellsworth 1 , Francisco Gonzalez Bobes 2 , Arvind Mathur 1 , James Kempson 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-01-02 , DOI: 10.1021/acs.oprd.3c00433 Xiaoping Hou 1 , Lucas W. Hernandez 2 , Elizabeth A. Jurica 1 , Rulin Zhao 1 , Bei Wang 1 , Michael Wong 1 , Jung-Hui Sun 1 , Dawn Sun 1 , Dauh-Rurng Wu 1 , Changxia Yuan 2 , Michael Hay 2 , Miao Yu 2 , Ximao Wu 1 , Yanting Huang 1 , Bruce A. Ellsworth 1 , Francisco Gonzalez Bobes 2 , Arvind Mathur 1 , James Kempson 1
Affiliation
|
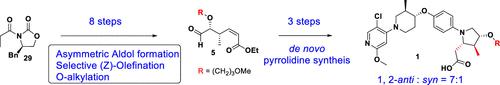
Herein we describe a series of synthetic efforts to prepare an advanced GPR40 agonist (compound 1), with focus on phase-appropriate processes that circumvented key reagents with short supply in the original synthesis. The key transformations refined for large-scale production were an asymmetric aldol reaction, O-alkylation of an unstable intermediate, selective (Z)-olefination, and reduction of a Weinreb amide to aldehyde. Additionally, the new route circumvented stability issues of the core pyrrolidine fragment through de novo synthesis, achieving high d.r. during ring formation. The new improved route was efficiently scaled up to prepare more than 100 g API for toxicology studies.
中文翻译:

先进 GPR40 激动剂合成过程的演变
在此,我们描述了一系列制备先进 GPR40 激动剂(化合物1)的合成工作,重点是避开原始合成中供应短缺的关键试剂的适相工艺。大规模生产的关键转化是不对称羟醛反应、不稳定中间体的O-烷基化、选择性 ( Z )-烯化以及 Weinreb 酰胺还原为醛。此外,新路线通过从头合成避免了核心吡咯烷片段的稳定性问题,在环形成过程中实现了高dr。新的改进路线被有效地扩大规模,以制备超过 100 g API 用于毒理学研究。
更新日期:2024-01-02
中文翻译:

先进 GPR40 激动剂合成过程的演变
在此,我们描述了一系列制备先进 GPR40 激动剂(化合物1)的合成工作,重点是避开原始合成中供应短缺的关键试剂的适相工艺。大规模生产的关键转化是不对称羟醛反应、不稳定中间体的O-烷基化、选择性 ( Z )-烯化以及 Weinreb 酰胺还原为醛。此外,新路线通过从头合成避免了核心吡咯烷片段的稳定性问题,在环形成过程中实现了高dr。新的改进路线被有效地扩大规模,以制备超过 100 g API 用于毒理学研究。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号