当前位置:
X-MOL 学术
›
ACS Macro Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sequence-Defined Tertiary Amine-Based Oligomer Employing a Scalable, Support-Free, and Protection/Deprotection-Free Iterative Strategy
ACS Macro Letters ( IF 5.1 ) Pub Date : 2024-01-02 , DOI: 10.1021/acsmacrolett.3c00589 Debashis Barik 1 , Mintu Porel 1, 2
ACS Macro Letters ( IF 5.1 ) Pub Date : 2024-01-02 , DOI: 10.1021/acsmacrolett.3c00589 Debashis Barik 1 , Mintu Porel 1, 2
Affiliation
|
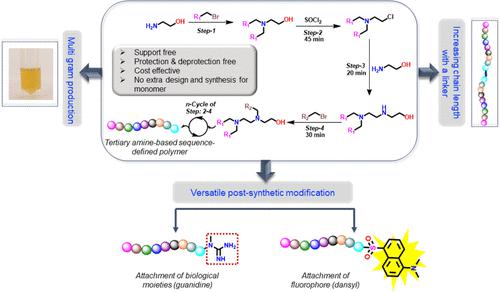
Sequence-defined oligomers (SDOs) with their unique monomeric sequence and customizable nature are attracting the attention of researchers globally. The structural and functional diversity attainable in SDOs makes this platform promising, albeit with challenges in the synthesis. Herein, we report the design and synthesis of a novel class of SDO by incorporating tertiary amines into the backbone from commercially available inexpensive materials. Tertiary amines were selected due to their various material and biomedical applications. Even though the synthesis and purification of amine compounds are challenging, their various significant applications, such as pharmaceuticals, catalysts, surfactants, corrosion inhibitors, dye intermediates, polymer additives, rubber accelerators, gas treating agents, agriculture, and analytical chemistry, make them fascinating. The synthetic strategy that is designed here is extremely efficient and economical for the scalable synthesis of the SDO and is support-free, protection–deprotection chemistry-free, and catalyst/template-free. Most importantly, no extra design and synthesis of the monomer is required here. The key reactions employed for the SDO synthesis are (i) transformation of the hydroxy group to a halide and (ii) substitution of the halide by the secondary amine units. Including the purifying processes, the multigram synthesis of 4-mer was completed in 12–14 h. The synthetic strategy was established by synthesizing two different sequences of SDOs. The SDOs are characterized by 1H NMR and LC-MS. The tandem MS (MS/MS) experiment was conducted in order to validate the sequences over the SDO chain. Furthermore, the SDO platform was advanced in two ways: (i) by increasing the chain length via attaching a linker, which provides a rapid method for increasing the tertiary amine over the SDO chain, and (ii) postsynthetic modification of SDO with other functional groups, including guanidine for biological importance and a well-known fluorophore dansyl group for material significance.
中文翻译:

采用可扩展、无支持和无保护/去保护迭代策略的序列定义的叔胺低聚物
序列定义的寡聚物(SDO)以其独特的单体序列和可定制的性质吸引了全球研究人员的关注。 SDO 可实现的结构和功能多样性使该平台充满希望,尽管在合成过程中存在挑战。在此,我们报告了通过将叔胺掺入市售廉价材料的主链中来设计和合成一类新型 SDO。选择叔胺是由于其各种材料和生物医学应用。尽管胺化合物的合成和纯化具有挑战性,但它们的各种重要应用,如药物、催化剂、表面活性剂、缓蚀剂、染料中间体、聚合物添加剂、橡胶促进剂、气体处理剂、农业和分析化学,使其令人着迷。这里设计的合成策略对于 SDO 的可扩展合成来说非常有效和经济,并且无需载体、无需保护-去保护化学、无需催化剂/模板。最重要的是,这里不需要额外的单体设计和合成。 SDO 合成所采用的关键反应是 (i) 将羟基转化为卤化物,以及 (ii) 用仲胺单元取代卤化物。包括纯化过程,4 聚体的多克合成在 12-14 小时内完成。通过合成两个不同的SDO序列建立了合成策略。 SDO 通过1 H NMR 和 LC-MS 进行表征。进行串联 MS (MS/MS) 实验是为了验证 SDO 链上的序列。 此外,SDO 平台通过两种方式进行了改进:(i)通过连接连接体增加链长度,这提供了一种快速方法来增加 SDO 链上的叔胺,以及(ii)用其他功能性物质对 SDO 进行合成后修饰基团,包括具有生物学重要性的胍和具有物质重要性的众所周知的荧光团丹酰基。
更新日期:2024-01-02
中文翻译:

采用可扩展、无支持和无保护/去保护迭代策略的序列定义的叔胺低聚物
序列定义的寡聚物(SDO)以其独特的单体序列和可定制的性质吸引了全球研究人员的关注。 SDO 可实现的结构和功能多样性使该平台充满希望,尽管在合成过程中存在挑战。在此,我们报告了通过将叔胺掺入市售廉价材料的主链中来设计和合成一类新型 SDO。选择叔胺是由于其各种材料和生物医学应用。尽管胺化合物的合成和纯化具有挑战性,但它们的各种重要应用,如药物、催化剂、表面活性剂、缓蚀剂、染料中间体、聚合物添加剂、橡胶促进剂、气体处理剂、农业和分析化学,使其令人着迷。这里设计的合成策略对于 SDO 的可扩展合成来说非常有效和经济,并且无需载体、无需保护-去保护化学、无需催化剂/模板。最重要的是,这里不需要额外的单体设计和合成。 SDO 合成所采用的关键反应是 (i) 将羟基转化为卤化物,以及 (ii) 用仲胺单元取代卤化物。包括纯化过程,4 聚体的多克合成在 12-14 小时内完成。通过合成两个不同的SDO序列建立了合成策略。 SDO 通过1 H NMR 和 LC-MS 进行表征。进行串联 MS (MS/MS) 实验是为了验证 SDO 链上的序列。 此外,SDO 平台通过两种方式进行了改进:(i)通过连接连接体增加链长度,这提供了一种快速方法来增加 SDO 链上的叔胺,以及(ii)用其他功能性物质对 SDO 进行合成后修饰基团,包括具有生物学重要性的胍和具有物质重要性的众所周知的荧光团丹酰基。































 京公网安备 11010802027423号
京公网安备 11010802027423号