当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Manufacturing Process for 6-Bromo-N,N-bis(4-methoxybenzyl)-4-methyl-5-(trifluoromethyl)pyridin-2-amine, a Key Intermediate in the Synthesis of KRAS G12C Inhibitor Divarasib
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-12-29 , DOI: 10.1021/acs.oprd.3c00366 Jeff Shen 1 , Nicholas A. White 1 , Qingping Tian 1 , Lauren E. Sirois 1 , Haiming Zhang 1 , Francis Gosselin 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-12-29 , DOI: 10.1021/acs.oprd.3c00366 Jeff Shen 1 , Nicholas A. White 1 , Qingping Tian 1 , Lauren E. Sirois 1 , Haiming Zhang 1 , Francis Gosselin 1
Affiliation
|
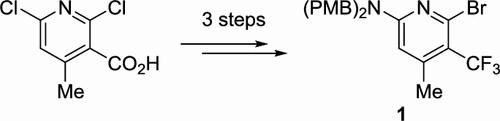
The densely functionalized heterocycle 6-bromo-N,N-bis(4-methoxybenzyl)-4-methyl-5-(trifluoromethyl)pyridin-2-amine (1) represents a key intermediate in the atroposelective synthesis of the potent KRAS G12C covalent inhibitor divarasib (GDC-6036). The first-generation manufacturing process of 1 comprised 9 steps, including tedious protecting group manipulations and a superstoichiometric copper-mediated trifluoromethylation of the corresponding iodopyridine using Chen’s reagent. Additional process research and development enabled an improved, scalable second-generation route furnishing 1 in only 3 steps from the readily available and inexpensive starting material 2,6-dichloro-4-methylnicotinic acid (13) via a deoxofluorination, a chlorine-to-bromine halogen exchange, and a regioselective SNAr amination.
中文翻译:

KRAS G12C 抑制剂 Divarasib 合成的关键中间体 6-溴-N,N-双(4-甲氧基苄基)-4-甲基-5-(三氟甲基)吡啶-2-胺的制造工艺
密集功能化杂环 6-溴-N , N-双(4-甲氧基苄基)-4-甲基-5-(三氟甲基)吡啶-2-胺 ( 1 ) 是强效 KRAS G12C 共价键的逆向选择性合成中的关键中间体抑制剂 Divarasib (GDC-6036)。1的第一代制造工艺包括9个步骤,包括繁琐的保护基操作和使用Chen试剂对相应的碘吡啶进行超化学计量的铜介导的三氟甲基化。额外的工艺研究和开发实现了改进的、可扩展的第二代路线,只需3 个步骤即可从容易获得且廉价的起始材料 2,6-二氯-4-甲基烟酸 ( 13 ) 通过脱氧氟化、氯转化为溴卤素交换和区域选择性 S N Ar 胺化。
更新日期:2023-12-29
中文翻译:

KRAS G12C 抑制剂 Divarasib 合成的关键中间体 6-溴-N,N-双(4-甲氧基苄基)-4-甲基-5-(三氟甲基)吡啶-2-胺的制造工艺
密集功能化杂环 6-溴-N , N-双(4-甲氧基苄基)-4-甲基-5-(三氟甲基)吡啶-2-胺 ( 1 ) 是强效 KRAS G12C 共价键的逆向选择性合成中的关键中间体抑制剂 Divarasib (GDC-6036)。1的第一代制造工艺包括9个步骤,包括繁琐的保护基操作和使用Chen试剂对相应的碘吡啶进行超化学计量的铜介导的三氟甲基化。额外的工艺研究和开发实现了改进的、可扩展的第二代路线,只需3 个步骤即可从容易获得且廉价的起始材料 2,6-二氯-4-甲基烟酸 ( 13 ) 通过脱氧氟化、氯转化为溴卤素交换和区域选择性 S N Ar 胺化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号