European Polymer Journal ( IF 5.8 ) Pub Date : 2023-12-30 , DOI: 10.1016/j.eurpolymj.2023.112721 Silja Boner , Kostas Parkatzidis , Nethmi De Alwis Watuthanthrige , Athina Anastasaki
|
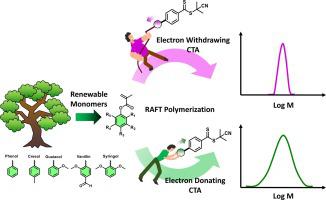
The use of renewable polymer feedstocks has attracted significant attention recently due to increased concerns about petrochemical-based materials and limited fossil-based resources. As a promising candidate, lignin-derived monomers have been polymerized via controlled radical polymerization to yield bio-based materials. Arguably, the most commonly employed polymerization methodology is reversible-addition-fragmentation chain-transfer (RAFT) polymerization which utilizes, almost exclusively, 2-cyano-2-propyl benzodithioate as a chain transfer agent (CTA). Despite the robustness of this approach, polymerization of lignin-derived methacrylates is far more challenging than of the commonly used monomers (e.g. methyl methacrylate), often resulting in polymers with moderate control over the molecular weight and dispersity (Đ). To improve control over the RAFT polymerization, we investigate here a series of 2-cyano-2-propyl benzodithioate derivatives, where the electronic properties of the Z group were systematically altered. The introduction of electron-withdrawing groups in the para-position of the phenyl ring of the Z group increased control over the polymerization by improving the chain transfer coefficient of the CTA, resulting in enhanced control over molecular weights and lower Đs. Conversely, the incorporation of electron-donating groups had the opposite effect. 2-cyano-2-propyl 4-cyanobenzodithioate was found to be the optimal CTA for the polymerization of a series of lignin-derived monomers resulting in Đs < 1.25. The robustness of the system was highlighted by the synthesis of higher molecular weights polymers and the efficient chain-extension of macroCTAs; the latter case yielding bio-based block copolymers with a Đ as low as 1.16. This work highlights that RAFT polymerization can, upon judicious optimization, be successfully employed for the synthesis of renewable polymers.
中文翻译:

可再生单体与二硫代苯甲酸酯的 RAFT 聚合:Z 基团取代基和反应条件的影响
由于人们对石化材料和有限化石资源的担忧增加,可再生聚合物原料的使用最近引起了极大的关注。作为一种有前途的候选者,木质素衍生单体已通过受控自由基聚合进行聚合以产生生物基材料。可以说,最常用的聚合方法是可逆加成断裂链转移 (RAFT) 聚合,它几乎专门使用 2-氰基-2-丙基苯并二硫酸酯作为链转移剂 (CTA)。尽管这种方法很稳健,但木质素衍生的甲基丙烯酸酯的聚合比常用单体(例如甲基丙烯酸甲酯)更具挑战性,通常会产生对分子量和分散性(Đ)具有适度控制的聚合物。为了改善对 RAFT 聚合的控制,我们研究了一系列 2-氰基-2-丙基苯并二硫代酸酯衍生物,其中 Z 基团的电子特性被系统地改变。在Z基团苯环的对位引入吸电子基团,通过提高CTA的链转移系数来增强对聚合的控制,从而增强对分子量的控制和更低的Đ s。相反,给电子基团的掺入则产生相反的效果。2-氰基-2-丙基 4-氰基苯并二硫代酯被发现是一系列木质素衍生单体聚合的最佳 CTA,导致Đ s < 1.25。高分子量聚合物的合成和大分子 CTA 的有效扩链凸显了该系统的稳健性;后一种情况产生Đ低至 1.16 的生物基嵌段共聚物。这项工作强调,经过明智的优化,RAFT 聚合可以成功地用于可再生聚合物的合成。






























 京公网安备 11010802027423号
京公网安备 11010802027423号