Acta Biomaterialia ( IF 9.4 ) Pub Date : 2023-12-28 , DOI: 10.1016/j.actbio.2023.12.040 Yuan Huang 1 , Bo Huang 1 , Dong Ye 2 , Xinxin Luo 1 , Xilin Xiong 1 , Huayu Xiong 1 , Hangxing Wang 1 , Qichao Zou 1 , Jichao Liang 1 , Suxiao Wang 1 , Limin Wu 3
|
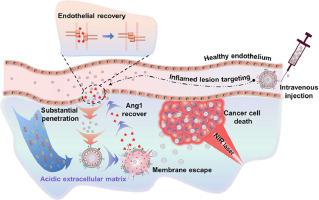
Nano-induced endothelial leakiness (NanoEL) can improve the ability of nanoparticles (NPs) to enter the tumor environment, nevertheless, it can inadvertently trigger adverse effects such as tumor metastasis. To overcome these concerns, it becomes important to develop a NPs design strategy that capitalizes on the NanoEL effect while averting unwanted side effects during the drug delivery process. Herein, we introduce the PLGA-ICG-PEI-Ang1@M NP which has a core comprising poly (lactic-co-glycolic acid) (PLGA) and the inner shell with a highly positively charged polyethyleneimine (PEI) and the anti-permeability growth factor Angiopoietin 1 (Ang1), while the outer shell is camouflaged with a Jurkat cell membrane. During the drug delivery process, our NPs exhibit their capability to selectively target and penetrate endothelial cell layers. Once the NPs penetrate the endothelial layer, the proton sponge effect triggered by PEI in the acidic environment surrounding the tumor site can rupture the cell membrane on the NPs' surface. This rupture, in turn, enables the positively charged Ang1 to be released due to the electrostatic repulsion from PEI and the disrupted endothelial layer can be restored. Consequently, the designed NPs can penetrate endothelial layers, promote the cell layer recovery, restrict the tumor metastasis, and facilitate efficient cancer therapy.
Statement of significance
- •
This work develops a NP design strategy that capitalizes on the nano-induced endothelial leakiness (NanoEL) effect while averting unwanted side effects such as tumor metastasis during the drug delivery process.
- •
The rational design of core-double shell PLGA-ICG-PEI-Ang1@M NP enables enhanced drug transport across the endothelial barrier inherited from both membrane-derived tumor homing and NanoEL effect brought by NPs.
- •
The controlled release of angiopoietin-1 in response to the tumor microenvironment allows a timely recovery of endothelial cell leakiness which can avert unwanted side effects due to the uncontrolled and persistent leakiness.
- •
The designed NPs can penetrate endothelial layers, expedite cell layer recovery, curtail tumor metastasis, and facilitate efficient cancer therapy.
中文翻译:

纳米诱导的内皮渗漏逆转纳米颗粒用于内皮细胞屏障的靶向、渗透和恢复
纳米诱导的内皮渗漏(NanoEL)可以提高纳米粒子(NP)进入肿瘤环境的能力,然而,它可能无意中引发肿瘤转移等不良反应。为了克服这些问题,开发一种纳米颗粒设计策略变得非常重要,该策略利用 NanoEL 效应,同时避免药物输送过程中不必要的副作用。在此,我们介绍了PLGA-ICG-PEI-Ang1@M NP,其核心由聚乳酸-乙醇酸共聚物(PLGA)组成,内壳为带高正电的聚乙烯亚胺(PEI),并且具有抗渗透性生长因子血管生成素 1 (Ang1),而外壳则用 Jurkat 细胞膜伪装。在药物输送过程中,我们的纳米粒子表现出选择性靶向和穿透内皮细胞层的能力。一旦纳米颗粒穿透内皮层,PEI在肿瘤部位周围的酸性环境中引发的质子海绵效应可以使纳米颗粒表面的细胞膜破裂。这种破裂反过来又使带正电的 Ang1 由于 PEI 的静电排斥而被释放,并且破坏的内皮层可以得到恢复。因此,设计的纳米颗粒可以穿透内皮层,促进细胞层恢复,限制肿瘤转移,促进有效的癌症治疗。
重要性声明
- •
这项工作开发了一种 NP 设计策略,该策略利用纳米诱导的内皮渗漏 (NanoEL) 效应,同时避免药物输送过程中出现肿瘤转移等不良副作用。 - •
核-双壳PLGA-ICG-PEI-Ang1@M NP的合理设计能够增强药物穿过内皮屏障的转运,继承了膜源性肿瘤归巢和NP带来的NanoEL效应。 - •
血管生成素-1响应肿瘤微环境而受控释放,可以及时恢复内皮细胞渗漏,从而避免由于不受控制和持续渗漏而产生的不良副作用。 - •
设计的纳米颗粒可以穿透内皮层,加速细胞层恢复,减少肿瘤转移,并促进有效的癌症治疗。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号