当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiscale elasticity mapping of biological samples in 3D at optical resolution
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2023-12-29 , DOI: 10.1016/j.actbio.2023.12.036
Kathryn Regan 1 , Robert LeBourdais 1 , Rohin Banerji 1 , Sue Zhang 1 , Johnathan Muhvich 1 , Siyi Zheng 1 , Hadi T Nia 1
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2023-12-29 , DOI: 10.1016/j.actbio.2023.12.036
Kathryn Regan 1 , Robert LeBourdais 1 , Rohin Banerji 1 , Sue Zhang 1 , Johnathan Muhvich 1 , Siyi Zheng 1 , Hadi T Nia 1
Affiliation
|
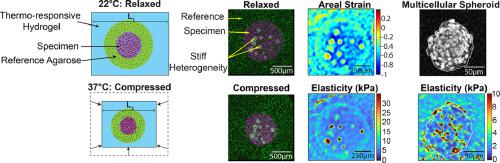
The mechanical properties of biological tissues have emerged as an integral determinant of tissue function in health and disease. Nonetheless, characterizing the elasticity of biological samples in 3D and at high resolution remains challenging. Here, we present a µElastography platform: a scalable elastography system that maps the elastic properties of tissues from cellular to organ scales. The platform leverages the use of a biocompatible, thermo-responsive hydrogel to deliver compressive stress to a biological sample and track its resulting deformation. By surrounding the specimen with a reference hydrogel of known Young's modulus, we are able to map the absolute values of elastic properties in biological samples. We validate the experimental and computational components of the platform using a hydrogel phantom and verify the system's ability to detect internal mechanical heterogeneities. We then apply the platform to map the elasticity of multicellular spheroids and the murine lymph node. With these applications, we demonstrate the platform's ability to map tissue elasticity at internal planes of interest, as well as capture mechanical heterogeneities neglected by most macroscale characterization techniques. The µElastography platform, designed to be implementable in any biology lab with access to 3D microscopy (e.g., confocal, multiphoton, or optical coherence microscopy), will provide the capability to characterize the mechanical properties of biological samples to labs across the large community of biological sciences by eliminating the need of specialized instruments such as atomic force microscopy. Understanding the elasticity of biological tissues is of great importance, but characterizing these properties typically requires highly specialized equipment. Utilizing stimulus-responsive hydrogels, we present a scalable, hydrogel-based elastography method that uses readily available reagents and imaging modalities to generate resolved maps of internal elasticity within biomaterials and biological samples at optical resolution. This new approach is capable of detecting internal stiffness heterogeneities within the 3D bulk of samples and is highly scalable across both imaging modalities and biological length scales. Thus, it will have significant impact on the measurement capabilities of labs studying engineered biomaterials, mechanobiology, disease progression, and tissue engineering and development.
中文翻译:

以光学分辨率对生物样品进行 3D 多尺度弹性映射
生物组织的机械性能已成为组织和疾病中组织功能不可或缺的决定因素。尽管如此,以 3D 和高分辨率表征生物样品的弹性仍然具有挑战性。在这里,我们介绍了一个 μElastography 平台:一种可扩展的弹性成像系统,可将组织的弹性特性从细胞尺度映射到器官尺度。该平台利用生物相容性、热响应性水凝胶向生物样品传递压缩应力并跟踪其产生的变形。通过用已知杨氏模量的参考水凝胶包围样品,我们能够绘制生物样品中弹性特性的绝对值。我们使用水凝胶模型验证平台的实验和计算组件,并验证系统检测内部机械异质性的能力。然后,我们应用该平台来绘制多细胞球体和小鼠淋巴结的弹性。通过这些应用程序,我们展示了该平台在感兴趣的内部平面上绘制组织弹性的能力,以及捕获大多数宏观表征技术所忽略的机械异质性。μElastography 平台旨在可在任何可使用 3D 显微镜(例如共聚焦、多光子或光学相干显微镜)的生物实验室中实施,无需原子力显微镜等专用仪器,即可为大型生物科学社区的实验室提供表征生物样品机械性能的能力。了解生物组织的弹性非常重要,但表征这些特性通常需要高度专业化的设备。 利用刺激响应水凝胶,我们提出了一种可扩展的、基于水凝胶的弹性成像方法,该方法使用现成的试剂和成像方式以光学分辨率生成生物材料和生物样品内部弹性的解析图。这种新方法能够检测 3D 样品中的内部刚度异质性,并且在成像模式和生物长度尺度上都具有高度可扩展性。因此,它将对研究工程生物材料、机械生物学、疾病进展以及组织工程和开发的实验室的测量能力产生重大影响。
更新日期:2023-12-29
中文翻译:

以光学分辨率对生物样品进行 3D 多尺度弹性映射
生物组织的机械性能已成为组织和疾病中组织功能不可或缺的决定因素。尽管如此,以 3D 和高分辨率表征生物样品的弹性仍然具有挑战性。在这里,我们介绍了一个 μElastography 平台:一种可扩展的弹性成像系统,可将组织的弹性特性从细胞尺度映射到器官尺度。该平台利用生物相容性、热响应性水凝胶向生物样品传递压缩应力并跟踪其产生的变形。通过用已知杨氏模量的参考水凝胶包围样品,我们能够绘制生物样品中弹性特性的绝对值。我们使用水凝胶模型验证平台的实验和计算组件,并验证系统检测内部机械异质性的能力。然后,我们应用该平台来绘制多细胞球体和小鼠淋巴结的弹性。通过这些应用程序,我们展示了该平台在感兴趣的内部平面上绘制组织弹性的能力,以及捕获大多数宏观表征技术所忽略的机械异质性。μElastography 平台旨在可在任何可使用 3D 显微镜(例如共聚焦、多光子或光学相干显微镜)的生物实验室中实施,无需原子力显微镜等专用仪器,即可为大型生物科学社区的实验室提供表征生物样品机械性能的能力。了解生物组织的弹性非常重要,但表征这些特性通常需要高度专业化的设备。 利用刺激响应水凝胶,我们提出了一种可扩展的、基于水凝胶的弹性成像方法,该方法使用现成的试剂和成像方式以光学分辨率生成生物材料和生物样品内部弹性的解析图。这种新方法能够检测 3D 样品中的内部刚度异质性,并且在成像模式和生物长度尺度上都具有高度可扩展性。因此,它将对研究工程生物材料、机械生物学、疾病进展以及组织工程和开发的实验室的测量能力产生重大影响。

































 京公网安备 11010802027423号
京公网安备 11010802027423号