当前位置:
X-MOL 学术
›
Sci. Bull.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decoding silkworm spinning programmed by pH and metal ions
Science Bulletin ( IF 18.8 ) Pub Date : 2023-12-29 , DOI: 10.1016/j.scib.2023.12.050 Kai Song 1 , Yejing Wang 2 , Wenjie Dong 3 , Zhenzhen Li 2 , Qingyou Xia 4 , Ping Zhu 5 , Huawei He 6
Science Bulletin ( IF 18.8 ) Pub Date : 2023-12-29 , DOI: 10.1016/j.scib.2023.12.050 Kai Song 1 , Yejing Wang 2 , Wenjie Dong 3 , Zhenzhen Li 2 , Qingyou Xia 4 , Ping Zhu 5 , Huawei He 6
Affiliation
|
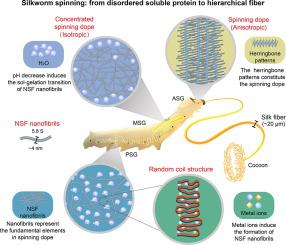
Silk is one of the toughest fibrous materials known despite spun at ambient temperature and pressure with water as a solvent. It is a great challenge to reproduce high-performance artificial fibers comparable to natural silk by bionic for the incomplete understanding of silkworm spinning . Here, we found that amphipol and digitonin stabilized the structure of natural silk fibroin (NSF) by a large-scale screening , and then studied the close-to-native ultrastructure and hierarchical assembly of NSF in the silk gland lumen. Our study showed that NSF formed reversible flexible nanofibrils mainly composed of random coils with a sedimentation coefficient of 5.8 S and a diameter of about 4 nm, rather than a micellar or rod-like structure assembled by the aggregation of globular NSF molecules. Metal ions were required for NSF nanofibril formation. The successive pH decrease from posterior silk gland (PSG) to anterior silk gland (ASG) resulted in a gradual increase in NSF hydrophobicity, thus inducing the sol-gelation transition of NSF nanofibrils. NSF nanofibrils were randomly dispersed from PSG to ASG-1, and self-assembled into anisotropic herringbone patterns at ASG-2 near the spinneret ready for silkworm spinning. Our findings reveal the controlled self-assembly mechanism of the multi-scale hierarchical architecture of NSF from nanofibrils to herringbone patterns programmed by metal ions and pH gradient, which provides novel insights into the spinning mechanism of silk-secreting animals and bioinspired design of high-performance fibers.
中文翻译:

破译由 pH 值和金属离子编程的蚕纺丝
尽管丝绸是在环境温度和压力下用水作为溶剂纺丝的,但它是已知的最坚韧的纤维材料之一。由于对蚕纺的认识不完全,利用仿生技术复制可与天然丝相媲美的高性能人造纤维是一个巨大的挑战。在这里,我们通过大规模筛选发现amphipol和洋地黄皂苷稳定了天然丝素蛋白(NSF)的结构,然后研究了NSF在丝腺腔内接近天然的超微结构和分层组装。我们的研究表明,NSF形成的可逆柔性纳米纤丝主要由沉降系数为5.8 S、直径约为4 nm的无规卷曲组成,而不是由球状NSF分子聚集而成的胶束或棒状结构。 NSF 纳米原纤维的形成需要金属离子。从后丝腺(PSG)到前丝腺(ASG)的pH值连续降低导致NSF疏水性逐渐增加,从而诱导NSF纳米原纤维的溶胶-凝胶化转变。 NSF纳米原纤维从PSG随机分散到ASG-1,并在喷丝头附近的ASG-2处自组装成各向异性人字形图案,准备用于蚕纺纱。我们的研究结果揭示了 NSF 多尺度分层结构的受控自组装机制,从纳米原纤维到由金属离子和 pH 梯度编程的人字形图案,这为丝分泌动物的旋转机制和高丝蛋白的仿生设计提供了新的见解。性能纤维。
更新日期:2023-12-29
中文翻译:

破译由 pH 值和金属离子编程的蚕纺丝
尽管丝绸是在环境温度和压力下用水作为溶剂纺丝的,但它是已知的最坚韧的纤维材料之一。由于对蚕纺的认识不完全,利用仿生技术复制可与天然丝相媲美的高性能人造纤维是一个巨大的挑战。在这里,我们通过大规模筛选发现amphipol和洋地黄皂苷稳定了天然丝素蛋白(NSF)的结构,然后研究了NSF在丝腺腔内接近天然的超微结构和分层组装。我们的研究表明,NSF形成的可逆柔性纳米纤丝主要由沉降系数为5.8 S、直径约为4 nm的无规卷曲组成,而不是由球状NSF分子聚集而成的胶束或棒状结构。 NSF 纳米原纤维的形成需要金属离子。从后丝腺(PSG)到前丝腺(ASG)的pH值连续降低导致NSF疏水性逐渐增加,从而诱导NSF纳米原纤维的溶胶-凝胶化转变。 NSF纳米原纤维从PSG随机分散到ASG-1,并在喷丝头附近的ASG-2处自组装成各向异性人字形图案,准备用于蚕纺纱。我们的研究结果揭示了 NSF 多尺度分层结构的受控自组装机制,从纳米原纤维到由金属离子和 pH 梯度编程的人字形图案,这为丝分泌动物的旋转机制和高丝蛋白的仿生设计提供了新的见解。性能纤维。































 京公网安备 11010802027423号
京公网安备 11010802027423号