当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interrupted Homolytic Substitution Enables Organoboron Compounds to Inhibit Radical Chain Reactions Rather than Initiate Them
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-12-29 , DOI: 10.1021/jacs.3c12438 Zijun Wu 1 , Robynne Vlaming 1 , Michael Donohoe 1 , Derek A Pratt 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-12-29 , DOI: 10.1021/jacs.3c12438 Zijun Wu 1 , Robynne Vlaming 1 , Michael Donohoe 1 , Derek A Pratt 1
Affiliation
|
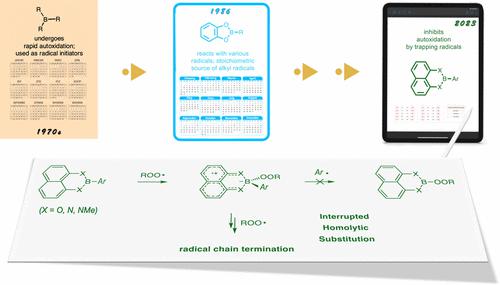
The reactions of organoboranes with peroxyl radicals are key to their use as radical initiators for a vast array of radical chain reactions, particularly at low temperatures where high stereoselectivity or regioselectivity is desired. Whereas these reactions generally proceed via concerted homolytic substitution (SH2) mechanisms, organoboranes that bear groups that can stabilize tetracoordinate boron radical “ate” complexes (e.g., catecholboranes) undergo this reaction via a stepwise addition/fragmentation sequence and serve as useful stoichiometric alkyl radical precursors. Here we show that arylboronic esters and amides derived from catecholborane and diaminonaphthaleneborane, respectively, are potent radical-trapping antioxidants (RTAs). Mechanistic studies reveal that this is because the radical “ate” complexes derived from peroxyl radical addition to boron are sufficiently persistent to trap another radical in an interrupted SH2 reaction. Remarkably, the reactivity of these organoboranes as inhibitors of autoxidation was shown to translate from simple hydrocarbons to the phospholipids of biological membranes such that they can inhibit ferroptosis, the cell death modality driven by lipid autoxidation and relevant in neurodegeneration and other major pathologies. The unique mechanism of these organoboranes is one of only a handful of RTA mechanisms that are not based on H-atom transfer processes and provide a new dimension to boron chemistry and its applications.
中文翻译:

中断的均解取代使有机硼化合物能够抑制自由基链式反应而不是引发它们
有机硼烷与过氧自由基的反应是其用作大量自由基链式反应的自由基引发剂的关键,特别是在需要高立体选择性或区域选择性的低温下。虽然这些反应通常通过一致的均解取代 (S H 2) 机制进行,但带有可稳定四配位硼自由基“ate”复合物的基团的有机硼烷(例如儿茶酚硼烷)通过逐步添加/断裂顺序进行该反应,并用作有用的化学计量烷基自由基前体。在这里,我们表明,分别衍生自儿茶酚硼烷和二氨基萘硼烷的芳基硼酸酯和酰胺是有效的自由基捕获抗氧化剂(RTA)。机理研究表明,这是因为过氧自由基与硼加成产生的自由基“吃”络合物足够持久,可以在中断的 S H 2 反应中捕获另一个自由基。值得注意的是,这些有机硼烷作为自动氧化抑制剂的反应性被证明可以从简单的碳氢化合物转化为生物膜的磷脂,从而可以抑制铁死亡,这是由脂质自动氧化驱动的细胞死亡方式,与神经变性和其他主要病理相关。这些有机硼烷的独特机制是少数不基于氢原子转移过程的 RTA 机制之一,为硼化学及其应用提供了新的维度。
更新日期:2023-12-29
中文翻译:

中断的均解取代使有机硼化合物能够抑制自由基链式反应而不是引发它们
有机硼烷与过氧自由基的反应是其用作大量自由基链式反应的自由基引发剂的关键,特别是在需要高立体选择性或区域选择性的低温下。虽然这些反应通常通过一致的均解取代 (S H 2) 机制进行,但带有可稳定四配位硼自由基“ate”复合物的基团的有机硼烷(例如儿茶酚硼烷)通过逐步添加/断裂顺序进行该反应,并用作有用的化学计量烷基自由基前体。在这里,我们表明,分别衍生自儿茶酚硼烷和二氨基萘硼烷的芳基硼酸酯和酰胺是有效的自由基捕获抗氧化剂(RTA)。机理研究表明,这是因为过氧自由基与硼加成产生的自由基“吃”络合物足够持久,可以在中断的 S H 2 反应中捕获另一个自由基。值得注意的是,这些有机硼烷作为自动氧化抑制剂的反应性被证明可以从简单的碳氢化合物转化为生物膜的磷脂,从而可以抑制铁死亡,这是由脂质自动氧化驱动的细胞死亡方式,与神经变性和其他主要病理相关。这些有机硼烷的独特机制是少数不基于氢原子转移过程的 RTA 机制之一,为硼化学及其应用提供了新的维度。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号