当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, Nematicidal Activity, and Mechanism of Novel Amide Derivatives Containing an 1,2,4-Oxadiazole Moiety
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2023-12-28 , DOI: 10.1021/acs.jafc.3c04945 Yu Wang 1 , Hongyi Song 1 , Sheng Wang 1 , Qingfeng Cai 1 , Jixiang Chen 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2023-12-28 , DOI: 10.1021/acs.jafc.3c04945 Yu Wang 1 , Hongyi Song 1 , Sheng Wang 1 , Qingfeng Cai 1 , Jixiang Chen 1
Affiliation
|
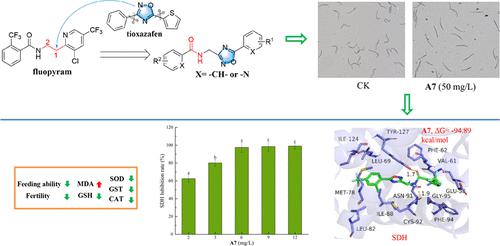
To discover new nematicides, a series of novel amide derivatives containing 1,2,4-oxadiazole were designed and synthesized. Several compounds showed excellent nematicidal activity. The LC50 values of compounds A7, A18, and A20–A22 against pine wood nematode (Bursaphelenchus xylophilus), rice stem nematode (Aphelenchoides besseyi), and sweet potato stem nematode (Ditylenchus destructor) were 1.39–3.09 mg/L, which were significantly better than the control nematicide tioxazafen (106, 49.0, and 75.0 mg/L, respectively). Compound A7 had an outstanding inhibitory effect on nematode feeding, reproductive ability, and egg hatching. Compound A7 effectively promoted the oxidative stress of nematodes and caused intestinal damage to nematodes. Compound A7 significantly inhibited the activity of succinate dehydrogenase (SDH) in nematodes, leading to blockage of electron transfer in the respiratory chain and thereby hindering the synthesis of adenosine triphosphate (ATP), which consequently affects the entire oxidative phosphorylation process to finally cause nematode death. Therefore, compound A7 can be used as a potential SDH inhibitor in nematicide applications.
中文翻译:

含1,2,4-恶二唑基团的新型酰胺衍生物的设计、合成、杀线虫活性及机理
为了发现新的杀线虫剂,设计并合成了一系列含有1,2,4-恶二唑的新型酰胺衍生物。几种化合物表现出优异的杀线虫活性。化合物A7 、 A18和A20 - A22对松材线虫( Bursaphelenchus xylophilus )、水稻茎线虫( Aphelenchoides besseyi )和甘薯茎线虫( Ditylenchus destructor )的LC 50值为1.39-3.09 mg/L,显着优于对照杀线虫剂噻唑芬(分别为 106、49.0 和 75.0 mg/L)。化合物A7对线虫摄食、繁殖能力、卵孵化具有显着的抑制作用。化合物A7有效促进线虫氧化应激,引起线虫肠道损伤。化合物A7显着抑制线虫体内琥珀酸脱氢酶(SDH)的活性,导致呼吸链电子传递受阻,从而阻碍三磷酸腺苷(ATP)的合成,从而影响整个氧化磷酸化过程,最终导致线虫死亡。因此,化合物A7可作为杀线虫剂应用中潜在的SDH抑制剂。
更新日期:2023-12-28
中文翻译:

含1,2,4-恶二唑基团的新型酰胺衍生物的设计、合成、杀线虫活性及机理
为了发现新的杀线虫剂,设计并合成了一系列含有1,2,4-恶二唑的新型酰胺衍生物。几种化合物表现出优异的杀线虫活性。化合物A7 、 A18和A20 - A22对松材线虫( Bursaphelenchus xylophilus )、水稻茎线虫( Aphelenchoides besseyi )和甘薯茎线虫( Ditylenchus destructor )的LC 50值为1.39-3.09 mg/L,显着优于对照杀线虫剂噻唑芬(分别为 106、49.0 和 75.0 mg/L)。化合物A7对线虫摄食、繁殖能力、卵孵化具有显着的抑制作用。化合物A7有效促进线虫氧化应激,引起线虫肠道损伤。化合物A7显着抑制线虫体内琥珀酸脱氢酶(SDH)的活性,导致呼吸链电子传递受阻,从而阻碍三磷酸腺苷(ATP)的合成,从而影响整个氧化磷酸化过程,最终导致线虫死亡。因此,化合物A7可作为杀线虫剂应用中潜在的SDH抑制剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号