当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Azepino[4,5-b]indole via Ring Expansion of Tetrahydro-β-carbolines Ammonium Ylide
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-12-29 , DOI: 10.1021/acs.joc.3c02249 Wang Shen 1 , Xiyao Lu 1 , Yu Shen 1 , Junjian Li 1 , Aiqun Jia 2 , Shi Tang 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-12-29 , DOI: 10.1021/acs.joc.3c02249 Wang Shen 1 , Xiyao Lu 1 , Yu Shen 1 , Junjian Li 1 , Aiqun Jia 2 , Shi Tang 1
Affiliation
|
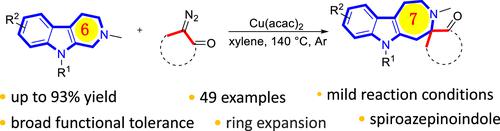
The formal cyclization strategy was generally used to construct azepino[4,5-b]indole. Herein, we reported a novel and expeditious protocol for the synthesis of quaternary carbon azepino[4,5-b]indole via ring expansion of ammonium ylide, which was formed by the reaction of tetrahydro-β-carbolines with the diazo compound. The easily available substrates, mild reaction conditions, and broad functional tolerance rendered this method a practical strategy that may significantly afford an efficient method of scaffold hopping in drug discovery.
中文翻译:

四氢-β-咔啉铵叶立德扩环合成氮杂[4,5-b]吲哚
正式的环化策略通常用于构建氮杂[4,5- b ]吲哚。在此,我们报道了一种通过四氢-β-咔啉与重氮化合物反应形成的铵叶立德扩环合成季碳氮杂[4,5- b ]吲哚的新颖且快速的方案。易于获得的底物、温和的反应条件和广泛的功能耐受性使该方法成为一种实用策略,可以在药物发现中显着提供有效的支架跳跃方法。
更新日期:2023-12-29
中文翻译:

四氢-β-咔啉铵叶立德扩环合成氮杂[4,5-b]吲哚
正式的环化策略通常用于构建氮杂[4,5- b ]吲哚。在此,我们报道了一种通过四氢-β-咔啉与重氮化合物反应形成的铵叶立德扩环合成季碳氮杂[4,5- b ]吲哚的新颖且快速的方案。易于获得的底物、温和的反应条件和广泛的功能耐受性使该方法成为一种实用策略,可以在药物发现中显着提供有效的支架跳跃方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号