当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bimolecular versus Trimolecular Reaction Pathways for H2O2 with Hypochlorous Species and Implications for Wastewater Reclamation
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-12-28 , DOI: 10.1021/acs.est.3c06375 Zonghao Luo 1, 2 , Wenjing Zhou 3 , Ying Jiang 1, 2 , Daisuke Minakata 4 , Richard Spinney 5 , Dionysios D Dionysiou 6 , Jianbo Liu 3 , Ruiyang Xiao 1, 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-12-28 , DOI: 10.1021/acs.est.3c06375 Zonghao Luo 1, 2 , Wenjing Zhou 3 , Ying Jiang 1, 2 , Daisuke Minakata 4 , Richard Spinney 5 , Dionysios D Dionysiou 6 , Jianbo Liu 3 , Ruiyang Xiao 1, 2
Affiliation
|
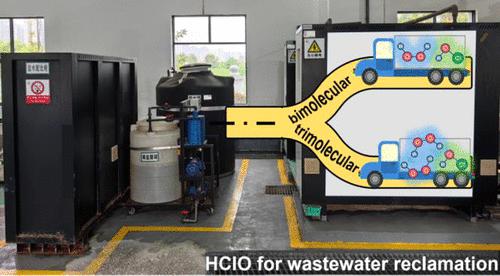
The benchmark advanced oxidation technology (AOT) that uses UV/H2O2 integrated with hypochlorous species exhibits great potential in removing micropollutants and enhancing wastewater treatability for reclamation purposes. Although efforts have been made to study the reactions of H2O2 with hypochlorous species, there exist great discrepancies in the order of reaction kinetics, the rate constants, and the molecule-level mechanisms. This results in an excessive use of hypochlorous reagents and system underperformance during treatment processes. Herein, the titled reaction was investigated systematically through complementary experimental and theoretical approaches. Stopped-flow spectroscopic measurements revealed a combination of bi- and trimolecular reaction kinetics. The bimolecular pathway dominates at low H2O2 concentrations, while the trimolecular pathway dominates at high H2O2 concentrations. Both reactions were simulated using direct dynamics trajectories, and the pathways identified in the trajectories were further validated by high-level quantum chemistry calculations. The theoretical results not only supported the spectroscopic data but also elucidated the molecule-level mechanisms and helped to address the origin of the discrepancies. In addition, the impact of the environmental matrix was evaluated by using two waters with discrete characteristics, namely municipal wastewater and ammonium-rich wastewater. Municipal wastewater had a negligible matrix effect on the reaction kinetics of H2O2 and the hypochlorous species, making it a highly suitable candidate for this integration technique. The obtained in-depth reaction mechanistic insights will enable the development of a viable and economical technology for safe water reuse.
中文翻译:

H2O2 与次氯酸的双分子与三分子反应途径及其对废水回收的影响
使用 UV/H 2 O 2与次氯酸物质相结合的基准高级氧化技术 (AOT) 在去除微污染物和提高废水可处理性以实现回收方面表现出巨大潜力。尽管人们已经努力研究H 2 O 2与次氯酸的反应,但在反应动力学顺序、速率常数和分子水平机制方面存在很大差异。这导致处理过程中次氯酸试剂的过度使用和系统性能不佳。在此,通过互补的实验和理论方法系统地研究了标题反应。停流光谱测量揭示了双分子和三分子反应动力学的组合。在低H 2 O 2浓度下双分子途径占主导地位,而在高H 2 O 2浓度下三分子途径占主导地位。这两个反应均使用直接动力学轨迹进行模拟,并且轨迹中确定的路径通过高级量子化学计算得到进一步验证。理论结果不仅支持了光谱数据,而且阐明了分子水平的机制,并有助于解决差异的根源。此外,还使用两种具有离散特征的水(即城市废水和富铵废水)评估了环境基质的影响。城市废水对 H 2 O 2和次氯酸物质的反应动力学的基质效应可以忽略不计,因此非常适合这种集成技术。 获得的深入反应机理见解将有助于开发可行且经济的安全水回用技术。
更新日期:2023-12-28
中文翻译:

H2O2 与次氯酸的双分子与三分子反应途径及其对废水回收的影响
使用 UV/H 2 O 2与次氯酸物质相结合的基准高级氧化技术 (AOT) 在去除微污染物和提高废水可处理性以实现回收方面表现出巨大潜力。尽管人们已经努力研究H 2 O 2与次氯酸的反应,但在反应动力学顺序、速率常数和分子水平机制方面存在很大差异。这导致处理过程中次氯酸试剂的过度使用和系统性能不佳。在此,通过互补的实验和理论方法系统地研究了标题反应。停流光谱测量揭示了双分子和三分子反应动力学的组合。在低H 2 O 2浓度下双分子途径占主导地位,而在高H 2 O 2浓度下三分子途径占主导地位。这两个反应均使用直接动力学轨迹进行模拟,并且轨迹中确定的路径通过高级量子化学计算得到进一步验证。理论结果不仅支持了光谱数据,而且阐明了分子水平的机制,并有助于解决差异的根源。此外,还使用两种具有离散特征的水(即城市废水和富铵废水)评估了环境基质的影响。城市废水对 H 2 O 2和次氯酸物质的反应动力学的基质效应可以忽略不计,因此非常适合这种集成技术。 获得的深入反应机理见解将有助于开发可行且经济的安全水回用技术。

































 京公网安备 11010802027423号
京公网安备 11010802027423号