当前位置:
X-MOL 学术
›
J. Mol. Liq.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effects of solvents on structure and energy of 6-amino-2-mercapto-3H-pyrimidin-4-one: A computational study
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2023-12-27 , DOI: 10.1016/j.molliq.2023.123821 Mohamed A. Abdel-Rahman , Shimaa Abdel Halim
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2023-12-27 , DOI: 10.1016/j.molliq.2023.123821 Mohamed A. Abdel-Rahman , Shimaa Abdel Halim
|
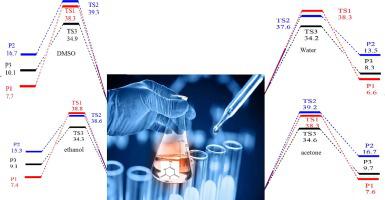
6-amino-2-mercapto-3H-pyrimidin-4-one (AMP) is a π-conjugated organic molecule extensively used as a semiconductor in many optoelectronic devices. The computational hybrid -generalized gradient Minnesota M06-2x, ωB97XD, and B3LYP functionals in conjunction with the correlation consistent polarized valence triplet zeta (cc-pVTZ) basis set and the moderate composite CBS-QB3 procedure were used with the aid of the Gaussian 09 program and the kinetic and statistical thermodynamical package (KiSThelP) in the temperature range 250–400 K to investigate the behavior of AMP in the gas phase, and in different polar (water, ethanol), and non-polar (dimethyl sulfoxide (DMSO), acetone)) solvents. Non-linear optical (NLO) characteristics and quantum chemical parameters were examined. Natural bond orbital analysis (NBO) was used to characterize the charge transfer of the electron density in the investigated compounds. The quality of the methods used was inspected and compared to give accurate results. The stability of AMP can be returned to the hyper conjugative interactions that leading to its nonlinear optical activity. Also, the charge delocalization was analyzed using natural bond orbital technique. The molecular electrostatic potential surfaces (MEPS) plots have been generated. The rate coefficients of tautomerization processes (k, in s) were calculated from the transition state theory (TST) and unimolecular Rice-Ramsperger-Kassel-Marcus (RRKM) methods. The results indicate comparable chemical rates obtained from TST and RRKM. In addition, positive temperature dependencies were located for all channels and a high dominance of transferring H-atom from the external N-atom to the S-atom (R3 reaction) during the applied temperature range which can return to its lower activation energy. This study is the first stone in studying AMP under different conditions such as the atmosphere, combustion conditions and as a nano catalyst.
中文翻译:

溶剂对 6-amino-2-mercapto-3H-pyrimidin-4-one 结构和能量的影响:计算研究
6-氨基-2-巯基-3H-嘧啶-4-酮 (AMP) 是一种 π 共轭有机分子,广泛用作许多光电器件中的半导体。在 Gaussian 09 的帮助下,使用计算混合广义梯度 Minnesota M06-2x、ωB97XD 和 B3LYP 泛函以及相关一致极化价三重态 zeta (cc-pVTZ) 基组和适度复合 CBS-QB3 程序程序和动力学和统计热力学软件包 (KiSThelP) 在 250–400 K 的温度范围内研究 AMP 在气相以及不同极性(水、乙醇)和非极性(二甲基亚砜 (DMSO))中的行为、丙酮))溶剂。检查了非线性光学(NLO)特性和量子化学参数。自然键轨道分析(NBO)用于表征所研究化合物中电子密度的电荷转移。对所用方法的质量进行了检查和比较,以给出准确的结果。 AMP 的稳定性可以恢复到导致其非线性光学活性的超共轭相互作用。此外,还使用自然键轨道技术分析了电荷离域。分子静电势面 (MEPS) 图已生成。互变异构过程的速率系数(k,单位为s)是根据过渡态理论(TST)和单分子Rice-Ramsperger-Kassel-Marcus(RRKM)方法计算的。结果表明,从 TST 和 RRKM 获得的化学速率相当。此外,所有通道都存在正温度依赖性,并且在所施加的温度范围内,H原子从外部N原子转移到S原子(R3反应)的高度优势,可以返回到其较低的活化能。这项研究是在不同条件下(例如大气、燃烧条件和作为纳米催化剂)研究 AMP 的第一块石头。
更新日期:2023-12-27
中文翻译:

溶剂对 6-amino-2-mercapto-3H-pyrimidin-4-one 结构和能量的影响:计算研究
6-氨基-2-巯基-3H-嘧啶-4-酮 (AMP) 是一种 π 共轭有机分子,广泛用作许多光电器件中的半导体。在 Gaussian 09 的帮助下,使用计算混合广义梯度 Minnesota M06-2x、ωB97XD 和 B3LYP 泛函以及相关一致极化价三重态 zeta (cc-pVTZ) 基组和适度复合 CBS-QB3 程序程序和动力学和统计热力学软件包 (KiSThelP) 在 250–400 K 的温度范围内研究 AMP 在气相以及不同极性(水、乙醇)和非极性(二甲基亚砜 (DMSO))中的行为、丙酮))溶剂。检查了非线性光学(NLO)特性和量子化学参数。自然键轨道分析(NBO)用于表征所研究化合物中电子密度的电荷转移。对所用方法的质量进行了检查和比较,以给出准确的结果。 AMP 的稳定性可以恢复到导致其非线性光学活性的超共轭相互作用。此外,还使用自然键轨道技术分析了电荷离域。分子静电势面 (MEPS) 图已生成。互变异构过程的速率系数(k,单位为s)是根据过渡态理论(TST)和单分子Rice-Ramsperger-Kassel-Marcus(RRKM)方法计算的。结果表明,从 TST 和 RRKM 获得的化学速率相当。此外,所有通道都存在正温度依赖性,并且在所施加的温度范围内,H原子从外部N原子转移到S原子(R3反应)的高度优势,可以返回到其较低的活化能。这项研究是在不同条件下(例如大气、燃烧条件和作为纳米催化剂)研究 AMP 的第一块石头。






































 京公网安备 11010802027423号
京公网安备 11010802027423号