当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Self-Adaptive Pyroptosis Inducer: Optimizing the Catalytic Microenvironment of Nanozymes by Membrane-Adhered Microbe Enables Potent Cancer Immunotherapy
Advanced Materials ( IF 27.4 ) Pub Date : 2023-12-28 , DOI: 10.1002/adma.202310063
Wenjie Wang 1, 2 , Lu Zhang 1, 2 , Yanjie Zhang 1, 2 , Xuemeng Liu 1, 2 , Anjun Song 1, 2 , Jinsong Ren 1, 2 , Xiaogang Qu 1, 2
Advanced Materials ( IF 27.4 ) Pub Date : 2023-12-28 , DOI: 10.1002/adma.202310063
Wenjie Wang 1, 2 , Lu Zhang 1, 2 , Yanjie Zhang 1, 2 , Xuemeng Liu 1, 2 , Anjun Song 1, 2 , Jinsong Ren 1, 2 , Xiaogang Qu 1, 2
Affiliation
|
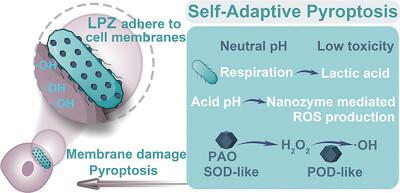
Pyroptosis has garnered increasing attention in cancer immunotherapy. Moreover, increasing plasma membrane damage by reactive oxygen species (ROS) is considered an effective strategy for promoting pyroptosis. However, the current tactics for enhancing membrane rupture in pyroptosis are limited by the inherent drawbacks of ROS and the immunosuppressive tumor microenvironment. Herein, a self-adaptive pyroptosis inducer (LPZ) is designed by integrating Lactobacillus rhamnosus GG (LGG) and an enzyme-like metal-organic framework to achieve potent pyroptosis immunotherapy. LPZ can adhere to cancer cell membranes through the interaction between the pili of LGG and the mucin of cancer cells. In particular, the adaptive formula can gradually enhance the ability of nanozymes to produce ROS by creating an acidic microenvironment through anaerobic respiration. These results verify that LPZ could generate high levels of ROS both on the membrane and within cancer cells, leading to pyroptotic cell death and strong antitumor immunity. Meanwhile, LGG are eventually killed by ROS in this process to halt their respiration and prevent potential biosafety concerns. Overall, this work provides new inspiration for the design of self-adaptive nanocatalytic drugs for cancer immunotherapy.
中文翻译:

自适应焦亡诱导剂:通过膜粘附微生物优化纳米酶的催化微环境,实现有效的癌症免疫治疗
细胞焦亡在癌症免疫治疗中引起了越来越多的关注。此外,活性氧(ROS)增加质膜损伤被认为是促进细胞焦亡的有效策略。然而,目前增强焦亡中膜破裂的策略受到ROS和免疫抑制肿瘤微环境的固有缺点的限制。在此,通过整合鼠李糖乳杆菌GG(LGG)和类酶金属有机框架设计了一种自适应焦亡诱导剂(LPZ),以实现有效的焦亡免疫治疗。 LPZ可以通过LGG的菌毛与癌细胞的粘蛋白之间的相互作用粘附在癌细胞膜上。特别是,适应性配方可以通过无氧呼吸创造酸性微环境,逐渐增强纳米酶产生ROS的能力。这些结果证实LPZ可以在癌细胞膜上和癌细胞内产生高水平的ROS,导致细胞焦亡和强大的抗肿瘤免疫力。与此同时,LGG 在此过程中最终被 ROS 杀死,以停止其呼吸并防止潜在的生物安全问题。总体而言,这项工作为癌症免疫治疗的自适应纳米催化药物的设计提供了新的灵感。
更新日期:2023-12-28
中文翻译:

自适应焦亡诱导剂:通过膜粘附微生物优化纳米酶的催化微环境,实现有效的癌症免疫治疗
细胞焦亡在癌症免疫治疗中引起了越来越多的关注。此外,活性氧(ROS)增加质膜损伤被认为是促进细胞焦亡的有效策略。然而,目前增强焦亡中膜破裂的策略受到ROS和免疫抑制肿瘤微环境的固有缺点的限制。在此,通过整合鼠李糖乳杆菌GG(LGG)和类酶金属有机框架设计了一种自适应焦亡诱导剂(LPZ),以实现有效的焦亡免疫治疗。 LPZ可以通过LGG的菌毛与癌细胞的粘蛋白之间的相互作用粘附在癌细胞膜上。特别是,适应性配方可以通过无氧呼吸创造酸性微环境,逐渐增强纳米酶产生ROS的能力。这些结果证实LPZ可以在癌细胞膜上和癌细胞内产生高水平的ROS,导致细胞焦亡和强大的抗肿瘤免疫力。与此同时,LGG 在此过程中最终被 ROS 杀死,以停止其呼吸并防止潜在的生物安全问题。总体而言,这项工作为癌症免疫治疗的自适应纳米催化药物的设计提供了新的灵感。































 京公网安备 11010802027423号
京公网安备 11010802027423号