当前位置:
X-MOL 学术
›
J. Mol. Struct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel 4,5-dibromo-N-phenyl-1H-pyrrole-2-carboxamide Hybrids as Promising DNA Gyrase Inhibitors: Design, synthesis and antimicrobial evaluation
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2023-12-29 , DOI: 10.1016/j.molstruc.2023.137359 Srinivas Reddy Merugu , Sithabile Mokoena , Vincent A. Obakachi , Baji Baba Shaik , Babita Kushawaha , Narva Deshwar Kushwaha , Blessing Wisdom Ike , Mahesh B. Palkar , Chandrakant G. Bonde , Ab Majeed Ganai , Ruchika Chauhan , Afsana Kajee , Meenu Ghai , Saqib Kidwai , Ramandeep Singh , Rajshekar Karpoormath
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2023-12-29 , DOI: 10.1016/j.molstruc.2023.137359 Srinivas Reddy Merugu , Sithabile Mokoena , Vincent A. Obakachi , Baji Baba Shaik , Babita Kushawaha , Narva Deshwar Kushwaha , Blessing Wisdom Ike , Mahesh B. Palkar , Chandrakant G. Bonde , Ab Majeed Ganai , Ruchika Chauhan , Afsana Kajee , Meenu Ghai , Saqib Kidwai , Ramandeep Singh , Rajshekar Karpoormath
|
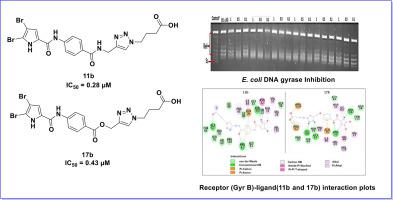
Bacterial remains a prominent and vital target in discovering new antibacterial drugs. In the present research work, we designed and synthesized a series of novel 4,5-dibromo-N-phenyl-1H-pyrrole-2-carboxamide hybrids containing substituted 1,2,3-triazole and isoxazole moieties which could serve as potential DNA gyrase inhibitors. The title compounds were synthesized with a good to excellent yield, and all the newly synthesized compounds were characterized by physicochemical and spectral analysis (FTIR, H NMR, and C NMR). The () inhibitory assay was performed for all synthesized compounds. Results showed that four compounds, (IC = 0.90 µM), (IC = 0.28 µM), (IC = 0.72 µM) and (IC = 0.43 µM), exhibited good inhibitory activity. molecular docking study was performed to understand the mode of interaction of compounds with the target enzyme. A docking study on the DNA gyrase protein revealed that compounds (-7.011 kcal/mol) and (-6.60 kcal/mol) interact with ARG136 and ASP73, two key amino acid residues, with docking scores of −7.01 and −6.60, respectively. The MD simulation was further employed to elucidate the thermodynamic binding energy, RMSD, and RMSF of compounds and . They exhibited binding energies of -47.598 kcal/mol and -41.682 kcal/mol, respectively. This provides valuable information on the binding mode and structure-activity relationship of these new hybrids of 4,5-dibromo-N-phenyl-1H-pyrrole-2-carboxamides as promising B inhibitors. In addition, these hybrid derivatives showed more prominent antibacterial action on Gram-negative rather than Gram-positive organisms. Surprisingly, compounds (MIC = 1.56 µg/mL) and (MIC = 3.125 µg/mL) also displayed promising anti-mycobacterial activity.
中文翻译:

新型 4,5-二溴-N-苯基-1H-吡咯-2-甲酰胺杂化物作为有前景的 DNA 旋转酶抑制剂:设计、合成和抗菌评估
细菌仍然是发现新抗菌药物的重要且重要的目标。在目前的研究工作中,我们设计并合成了一系列新型4,5-二溴-N-苯基-1H-吡咯-2-甲酰胺杂化物,其中含有取代的1,2,3-三唑和异恶唑部分,可作为潜在的DNA旋转酶抑制剂。标题化合物的合成具有良好至优异的收率,并且所有新合成的化合物均通过物理化学和光谱分析(FTIR、H NMR 和 C NMR)进行表征。对所有合成的化合物进行了()抑制测定。结果表明,四种化合物(IC = 0.90 µM)、(IC = 0.28 µM)、(IC = 0.72 µM)和(IC = 0.43 µM)表现出良好的抑制活性。进行分子对接研究以了解化合物与目标酶的相互作用模式。对DNA旋转酶蛋白的对接研究表明,化合物(-7.011 kcal/mol)和(-6.60 kcal/mol)与两个关键氨基酸残基ARG136和ASP73相互作用,对接分数分别为-7.01和-6.60。 MD 模拟进一步用于阐明化合物 和 的热力学结合能、RMSD 和 RMSF。它们的结合能分别为-47.598 kcal/mol和-41.682 kcal/mol。这为这些新型 4,5-二溴-N-苯基-1H-吡咯-2-甲酰胺杂化物作为有前景的 B 抑制剂的结合模式和构效关系提供了有价值的信息。此外,这些杂合衍生物对革兰氏阴性菌比革兰氏阳性菌表现出更显着的抗菌作用。令人惊讶的是,化合物 (MIC = 1.56 µg/mL) 和 (MIC = 3.125 µg/mL) 也表现出有希望的抗分枝杆菌活性。
更新日期:2023-12-29
中文翻译:

新型 4,5-二溴-N-苯基-1H-吡咯-2-甲酰胺杂化物作为有前景的 DNA 旋转酶抑制剂:设计、合成和抗菌评估
细菌仍然是发现新抗菌药物的重要且重要的目标。在目前的研究工作中,我们设计并合成了一系列新型4,5-二溴-N-苯基-1H-吡咯-2-甲酰胺杂化物,其中含有取代的1,2,3-三唑和异恶唑部分,可作为潜在的DNA旋转酶抑制剂。标题化合物的合成具有良好至优异的收率,并且所有新合成的化合物均通过物理化学和光谱分析(FTIR、H NMR 和 C NMR)进行表征。对所有合成的化合物进行了()抑制测定。结果表明,四种化合物(IC = 0.90 µM)、(IC = 0.28 µM)、(IC = 0.72 µM)和(IC = 0.43 µM)表现出良好的抑制活性。进行分子对接研究以了解化合物与目标酶的相互作用模式。对DNA旋转酶蛋白的对接研究表明,化合物(-7.011 kcal/mol)和(-6.60 kcal/mol)与两个关键氨基酸残基ARG136和ASP73相互作用,对接分数分别为-7.01和-6.60。 MD 模拟进一步用于阐明化合物 和 的热力学结合能、RMSD 和 RMSF。它们的结合能分别为-47.598 kcal/mol和-41.682 kcal/mol。这为这些新型 4,5-二溴-N-苯基-1H-吡咯-2-甲酰胺杂化物作为有前景的 B 抑制剂的结合模式和构效关系提供了有价值的信息。此外,这些杂合衍生物对革兰氏阴性菌比革兰氏阳性菌表现出更显着的抗菌作用。令人惊讶的是,化合物 (MIC = 1.56 µg/mL) 和 (MIC = 3.125 µg/mL) 也表现出有希望的抗分枝杆菌活性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号