当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Suppression of Hydrogen Evolution Reaction by Modulating the Surface Redox Potential Toward Long-Life Zinc Metal Anodes
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2023-12-28 , DOI: 10.1002/adfm.202312564 Jiajun Chen 1 , Jiaming Xiong 1 , Minghui Ye 1 , Zhipeng Wen 1 , Yufei Zhang 1 , Yongchao Tang 1 , Xiaoqing Liu 1 , Cheng Chao Li 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2023-12-28 , DOI: 10.1002/adfm.202312564 Jiajun Chen 1 , Jiaming Xiong 1 , Minghui Ye 1 , Zhipeng Wen 1 , Yufei Zhang 1 , Yongchao Tang 1 , Xiaoqing Liu 1 , Cheng Chao Li 1
Affiliation
|
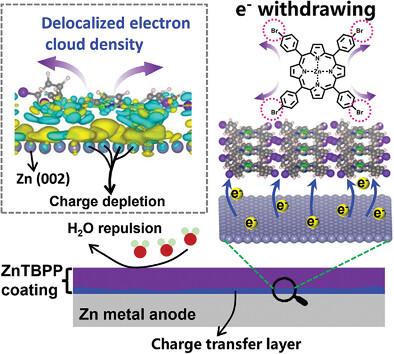
Aqueous Zn metal batteries hold significant promise for large-scale energy storage owing to their high safety and low cost. Nevertheless, severe corrosion and uncontrollable dendrite growth hinder the cyclic stability of Zn metal anodes. Herein, a porphyrin-Zn(II) (ZnTBPP) based multifunctional artificial layer to stabilize Zn anodes by suppression of hydrogen evolution reactions is designed. This hydrophobic interfacial layer repels active water molecules and facilitates the electron transfer from Zn interface to the ZnTBPP layer, thereby increasing the inherent hydrogen evolution potential of Zn anodes. Differential charge density maps reveal that the electron cloud density of the charge transfer layer within the ZnTBPP layer is delocalized due to the electron-withdrawing effect of Br-containing functional groups in the ZnTBPP ligands, depleting the interfacial electrons of Zn metal anodes. As a result, H* exhibits a higher Gibbs free energy (∆GH*) of 0.75 eV on Zn with a ZnTBPP coating layer in contrast to bare Zn (0.55 eV), effectively suppressing H2 production. Specifically, the H2 evolution rate of ZnTBPP@Zn (4.06 µmol h−1 cm−2) is ≈2.2 times lower than that of bare Zn. The ZnTBPP@Zn//NaV3O8·1.5H2O full cells exhibit an ultra-long cycling life of 10 000 cycles at 10 A g−1.
中文翻译:

通过调节表面氧化还原电位来抑制析氢反应以获得长寿命的锌金属阳极
水系锌金属电池由于其高安全性和低成本而在大规模储能方面具有重大前景。然而,严重的腐蚀和不可控的枝晶生长阻碍了锌金属阳极的循环稳定性。在此,设计了一种基于卟啉-Zn(II) (ZnTBPP) 的多功能人工层,通过抑制析氢反应来稳定锌阳极。这种疏水界面层排斥活性水分子,促进电子从 Zn 界面转移到 ZnTBPP 层,从而增加 Zn 阳极固有的析氢潜力。微分电荷密度图表明,由于ZnTBPP配体中含Br官能团的吸电子效应,ZnTBPP层内电荷转移层的电子云密度发生离域,耗尽了Zn金属阳极的界面电子。因此,与裸Zn (0.55 eV)相比,具有ZnTBPP涂层的Zn上的H*表现出更高的吉布斯自由能(ΔG H* ),为0.75 eV,从而有效抑制H 2的产生。具体而言, ZnTBPP@Zn(4.06 µmol h -1 cm -2 )的H 2析出速率比裸Zn低约2.2倍。 ZnTBPP@Zn//NaV 3 O 8 ·1.5H 2 O全电池在10 A g -1下表现出10 000次循环的超长循环寿命。
更新日期:2023-12-28
中文翻译:

通过调节表面氧化还原电位来抑制析氢反应以获得长寿命的锌金属阳极
水系锌金属电池由于其高安全性和低成本而在大规模储能方面具有重大前景。然而,严重的腐蚀和不可控的枝晶生长阻碍了锌金属阳极的循环稳定性。在此,设计了一种基于卟啉-Zn(II) (ZnTBPP) 的多功能人工层,通过抑制析氢反应来稳定锌阳极。这种疏水界面层排斥活性水分子,促进电子从 Zn 界面转移到 ZnTBPP 层,从而增加 Zn 阳极固有的析氢潜力。微分电荷密度图表明,由于ZnTBPP配体中含Br官能团的吸电子效应,ZnTBPP层内电荷转移层的电子云密度发生离域,耗尽了Zn金属阳极的界面电子。因此,与裸Zn (0.55 eV)相比,具有ZnTBPP涂层的Zn上的H*表现出更高的吉布斯自由能(ΔG H* ),为0.75 eV,从而有效抑制H 2的产生。具体而言, ZnTBPP@Zn(4.06 µmol h -1 cm -2 )的H 2析出速率比裸Zn低约2.2倍。 ZnTBPP@Zn//NaV 3 O 8 ·1.5H 2 O全电池在10 A g -1下表现出10 000次循环的超长循环寿命。






























 京公网安备 11010802027423号
京公网安备 11010802027423号