当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reconstitution of the Alzheimer’s Disease Tau Core Structure from Recombinant Tau297–391 Yields Variable Quaternary Structures as Seen by Negative Stain and Cryo-EM
Biochemistry ( IF 2.9 ) Pub Date : 2023-12-28 , DOI: 10.1021/acs.biochem.3c00425
Calina Glynn 1, 2 , Joshua E Chun 1, 2 , Cameron C Donahue 1, 2 , Monica J S Nadler 1, 2 , Zhanyun Fan 1, 2 , Bradley T Hyman 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2023-12-28 , DOI: 10.1021/acs.biochem.3c00425
Calina Glynn 1, 2 , Joshua E Chun 1, 2 , Cameron C Donahue 1, 2 , Monica J S Nadler 1, 2 , Zhanyun Fan 1, 2 , Bradley T Hyman 1, 2
Affiliation
|
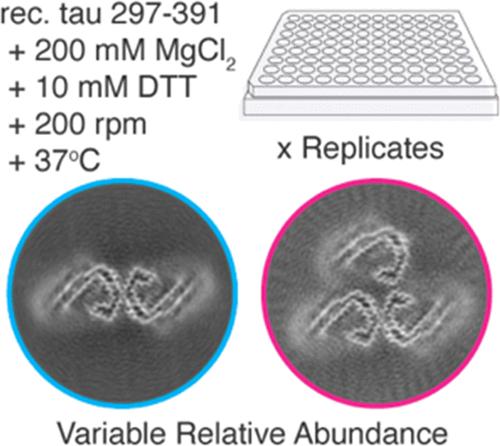
The protein tau misfolds into disease-specific fibrillar structures in more than 20 neurodegenerative diseases collectively referred to as tauopathies. To understand and prevent disease-specific mechanisms of filament formation, in vitro models for aggregation that robustly yield these different end point structures will be necessary. Here, we used cryo-electron microscopy (cryo-EM) to reconstruct fibril polymorphs taken on by residues 297–391 of tau under conditions previously shown to give rise to the core structure found in Alzheimer’s disease (AD). While we were able to reconstitute the AD tau core fold, the proportion of these paired helical filaments (PHFs) was highly variable, and a majority of filaments were composed of PHFs with an additional identical C-shaped protofilament attached near the PHF interface, termed triple helical filaments (THFs). Since the impact of filament layer quaternary structure on the biological properties of tau and other amyloid filaments is not known, the applications for samples of this morphology are presently uncertain. We further demonstrate the variation in the proportion of PHFs and PHF-like fibrils compared to other morphologies as a function of shaking time and AD polymorph-favoring cofactor concentration. This variation in polymorph abundance, even under identical experimental conditions, highlights the variation that can arise both within a lab and in different laboratory settings when reconstituting specific fibril polymorphs in vitro.
中文翻译:

从重组 Tau297-391 重建阿尔茨海默病 Tau 核心结构可产生可变的四元结构,如阴性染色和冷冻电镜所见
蛋白 tau 在 20 多种统称为 tau 蛋白病的神经退行性疾病中错误折叠成疾病特异性纤维结构。为了了解和防止细丝形成的疾病特异性机制,需要能够稳健地产生这些不同终点结构的体外聚集模型。在这里,我们使用冷冻电子显微镜 (cryo-EM) 重建了 tau 残基 297-391 所承担的原纤维多晶,在先前显示的条件下产生了阿尔茨海默病 (AD) 中发现的核心结构。虽然我们能够重建 AD tau 核心折叠,但这些成对螺旋丝 (PHF) 的比例变化很大,并且大多数细丝由 PHF 组成,在 PHF 界面附近连接着一个额外的相同的 C 形原丝,称为三螺旋丝 (THF)。由于细丝层四元结构对 tau 和其他淀粉样蛋白细丝的生物学特性的影响尚不清楚,因此这种形态的样品的应用目前尚不确定。我们进一步证明了与其他形态相比,PHFs 和 PHF 样原纤维比例的变化是振荡时间和有利于 AD 多晶型的辅因子浓度的函数。即使在相同的实验条件下,多晶型物丰度的这种变化也突出了在体外重构特定原纤维多晶型物时,实验室内和不同实验室环境中可能出现的变化。
更新日期:2023-12-28
中文翻译:

从重组 Tau297-391 重建阿尔茨海默病 Tau 核心结构可产生可变的四元结构,如阴性染色和冷冻电镜所见
蛋白 tau 在 20 多种统称为 tau 蛋白病的神经退行性疾病中错误折叠成疾病特异性纤维结构。为了了解和防止细丝形成的疾病特异性机制,需要能够稳健地产生这些不同终点结构的体外聚集模型。在这里,我们使用冷冻电子显微镜 (cryo-EM) 重建了 tau 残基 297-391 所承担的原纤维多晶,在先前显示的条件下产生了阿尔茨海默病 (AD) 中发现的核心结构。虽然我们能够重建 AD tau 核心折叠,但这些成对螺旋丝 (PHF) 的比例变化很大,并且大多数细丝由 PHF 组成,在 PHF 界面附近连接着一个额外的相同的 C 形原丝,称为三螺旋丝 (THF)。由于细丝层四元结构对 tau 和其他淀粉样蛋白细丝的生物学特性的影响尚不清楚,因此这种形态的样品的应用目前尚不确定。我们进一步证明了与其他形态相比,PHFs 和 PHF 样原纤维比例的变化是振荡时间和有利于 AD 多晶型的辅因子浓度的函数。即使在相同的实验条件下,多晶型物丰度的这种变化也突出了在体外重构特定原纤维多晶型物时,实验室内和不同实验室环境中可能出现的变化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号