Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2023-12-27 , DOI: 10.1016/j.bmc.2023.117581 Tomoyoshi Imaizumi 1 , Itsuro Shimada 1 , Yoshiki Satake 1 , Susumu Yamaki 1 , Takanori Koike 1 , Takahiro Nigawara 1 , Osamu Kaneko 1 , Yasushi Amano 1 , Kenichi Mori 1 , Yosuke Yamanaka 1 , Ayako Nakayama 1 , Yoshihiro Nishizono 1 , Masashi Shimazaki 1 , Takeyuki Nagashima 1 , Kazuyuki Kuramoto 1
|
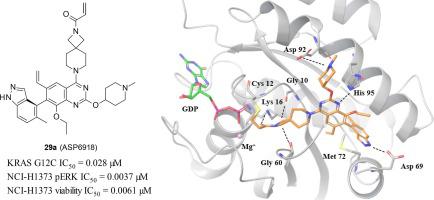
Although KRAS protein had been classified as an undruggable target, inhibitors of KRAS G12C mutant protein were recently reported to show clinical efficacy in solid tumors. In our previous report, we identified 1-{2,7-diazaspiro[3.5]non-2-yl}prop-2-en-1-one derivative (1) as a KRAS G12C inhibitor that covalently binds to Cys12 of KRAS G12C protein. Compound 1 exhibited potent cellular pERK inhibition and cell growth inhibition against a KRAS G12C mutation-positive cell line and showed an antitumor effect on subcutaneous administration in an NCI-H1373 (KRAS G12C mutation-positive cell line) xenograft mouse model in a dose-dependent manner. In this report, we further optimized the substituents on the quinazoline scaffold based on the structure-based drug design from the co-crystal structure analysis of compound 1 and KRAS G12C to enhance in vitro activity. As a result, ASP6918 was found to exhibit extremely potent in vitro activity and induce dose-dependent tumor regression in an NCI-H1373 xenograft mouse model after oral administration.
中文翻译:

ASP6918(一种 KRAS G12C 抑制剂)的发现:1-{2,7-diazaspiro[3.5]non-2-yl}prop-2-en-1-one 衍生物的合成和构效关系,作为具有良好效力和稳定性的共价抑制剂治疗实体瘤的口服活性
尽管 KRAS 蛋白已被归类为不可成药靶点,但最近有报道称 KRAS G12C 突变蛋白抑制剂在实体瘤中显示出临床疗效。在我们之前的报告中,我们鉴定出 1-{2,7-diazaspiro[3.5]non-2-yl}prop-2-en-1-one 衍生物 ( 1 ) 作为 KRAS G12C 抑制剂,可与 KRAS G12C 的 Cys12 共价结合蛋白质。化合物1对 KRAS G12C 突变阳性细胞系表现出有效的细胞 pERK 抑制和细胞生长抑制作用,并在剂量依赖性的NCI-H1373(KRAS G12C 突变阳性细胞系)异种移植小鼠模型中皮下给药时显示出抗肿瘤作用方式。在本报告中,我们通过化合物1和KRAS G12C的共晶结构分析,在基于结构的药物设计的基础上,进一步优化了喹唑啉支架上的取代基,以增强体外活性。结果发现,ASP6918 表现出极强的体外活性,并在口服给药后在 NCI-H1373 异种移植小鼠模型中诱导剂量依赖性肿瘤消退。































 京公网安备 11010802027423号
京公网安备 11010802027423号