Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2023-12-27 , DOI: 10.1016/j.jcis.2023.12.150 Yin Chen 1 , Lin Chen 2 , Yanfen Liao 1 , Zhuofan Chen 1 , Xiaoqian Ma 1
|
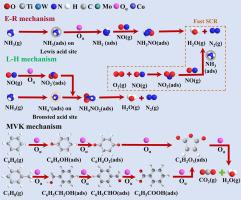
Previous studies have indicated the potential of monometallic-modified TiO2 catalysts in controlling nitrogen oxide (NOx) and volatile organic compounds (VOCs) in coal-fired flue gas. Unfortunately, increasing selective catalytic reduction (SCR) activity under complicated coal-fired flue gas status is tricky. In this study, modified Co-MoWTiO2 catalysts with multiple active sites were synthesized using the wet impregnation method, which exhibited excellent multi-pollution control ability of NO, benzene and toluene under low oxygen and high SO2 concentrations. The modification of Mo and Co achieved high dispersion and electron transfer. The interaction between W5+/W6+ and Co2+/Co3+ promoted gas-phase O2 adsorption on the catalyst surface, forming of reactive oxygen species (Oα). Density functional theory (DFT) calculations informed that the doping of Co effectively enhanced the NH3 and O2 adsorption capacity of the catalyst, and Co possessed the maximum adsorption energy for NH3 and O2. Possible pathways of multi-pollution control of NO, C6H6, and C7H8 were speculated. NH3/NH4+ on the Lewis/Bronsted acid site is reacted with intermediates of NO (e.g., NO2, nitrite, nitrate) via the Langmuir-Hinshelwood and Eley-Rideal mechanism. The introduction of NO and NH3 did not disrupt the oxidation pathways of benzene and toluene. Following the Mars-van Krevelen mechanism, C6H6 and C7H8 were progressively mineralized by Oα into CO2 and H2O.
中文翻译:

铜/镍/钴改性钼钨钛基催化剂用于氮氧化物、苯和甲苯多重污染控制:增强氧化还原能力及机理研究
先前的研究表明单金属改性TiO 2催化剂在控制燃煤烟气中的氮氧化物(NO x )和挥发性有机化合物(VOC)方面具有潜力。不幸的是,在复杂的燃煤烟气状态下提高选择性催化还原(SCR)活性是很棘手的。本研究采用湿法浸渍法合成了具有多活性位点的改性Co-MoWTiO 2催化剂,该催化剂在低氧和高SO 2浓度下表现出优异的NO、苯和甲苯多重污染控制能力。 Mo和Co的改性实现了高分散性和电子转移。 W 5+ /W 6+与Co 2+ /Co 3+之间的相互作用促进了催化剂表面气相O 2 的吸附,形成活性氧(O α )。密度泛函理论(DFT)计算表明,Co的掺杂有效增强了催化剂对NH 3和O 2的吸附能力,并且Co对NH 3和O 2具有最大的吸附能。推测了NO、C 6 H 6和C 7 H 8多重污染控制的可能途径。 Lewis/Bronsted酸位上的NH 3 /NH 4 +通过Langmuir-Hinshelwood和Eley-Rideal机理与NO中间体(例如NO 2 、亚硝酸盐、硝酸盐)反应。 NO和NH 3的引入没有破坏苯和甲苯的氧化途径。 按照Mars-van Krevelen机制,C 6 H 6和C 7 H 8逐渐被O α矿化成CO 2和H 2 O。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号