当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aminoquinoline-based Re(I) tricarbonyl complexes: Insights into their antiproliferative activity and mechanisms of action
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-12-27 , DOI: 10.1016/j.ejmech.2023.116094 Paige S Zinman 1 , Athi Welsh 1 , Reinner O Omondi 1 , Saif Khan 2 , Sharon Prince 2 , Ebbe Nordlander 3 , Gregory S Smith 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-12-27 , DOI: 10.1016/j.ejmech.2023.116094 Paige S Zinman 1 , Athi Welsh 1 , Reinner O Omondi 1 , Saif Khan 2 , Sharon Prince 2 , Ebbe Nordlander 3 , Gregory S Smith 1
Affiliation
|
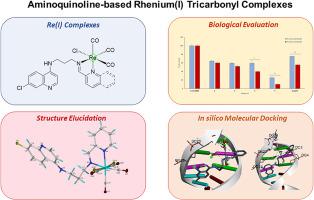
In an effort to develop new potent anticancer agents, two Schiff base rhenium(I) tricarbonyl complexes, containing the ubiquitous aminoquinoline scaffold, were synthesized. Both aminoquinoline ligands and Re(I) complexes showed adequate stability over a 48-h incubation period. Furthermore, the cytotoxic activity of the precursor ligands and rhenium(I) complexes were evaluated against the hormone-dependent MCF-7 and hormone-independent triple negative MDA-MB-231 breast cancer cell lines. Inclusion of the [Re(CO)Cl] entity significantly enhanced the cytotoxicity of the aminoquinoline Schiff base ligands against the tested cancer cell lines. Remarkably, the incorporation of the Schiff-base iminoquinolyl entity notably enhanced the cytotoxic activity of the Re(I) complexes, in comparison with the iminopyridyl entity. Notably, the quinolyl-substituted complex showed up to three-fold higher activity than cisplatin against breast cancer cell lines, underpinning the significance of the quinoline pharmacophore in rational drug design. In addition, the most active Re(I) complex showed better selectivity towards the breast cancer cells over non-tumorigenic FG-0 cells. Western blotting revealed that the complexes increased levels of γH2AX, a key DNA damage response protein. Moreover, apoptosis was confirmed in both cell lines due to the detection of cleaved PARP. The complexes show favourable binding affinities towards both calf thymus DNA (CT-DNA), and bovine serum albumin (BSA), and the order of their interactions align with their cytotoxic effects. The molecular simulations of the complexes were also performed with CT-DNA and BSA targets.
中文翻译:

基于氨基喹啉的 Re(I) 三羰基复合物:深入了解其抗增殖活性和作用机制
为了开发新的有效抗癌药物,合成了两种希夫碱铼(I)三羰基络合物,其中含有普遍存在的氨基喹啉支架。氨基喹啉配体和 Re(I) 复合物在 48 小时的孵育期内均表现出足够的稳定性。此外,还针对激素依赖性 MCF-7 和激素非依赖性三阴性 MDA-MB-231 乳腺癌细胞系评估了前体配体和铼 (I) 配合物的细胞毒活性。 [Re(CO)Cl]实体的包含显着增强了氨基喹啉席夫碱配体对测试的癌细胞系的细胞毒性。值得注意的是,与亚氨基吡啶基实体相比,席夫碱亚氨基喹啉基实体的掺入显着增强了 Re(I) 复合物的细胞毒活性。值得注意的是,喹啉基取代的复合物对乳腺癌细胞系的活性比顺铂高出三倍,支撑了喹啉药效团在合理药物设计中的重要性。此外,与非致瘤性 FG-0 细胞相比,最活跃的 Re(I) 复合物对乳腺癌细胞表现出更好的选择性。蛋白质印迹显示,复合物增加了 γH2AX(一种关键的 DNA 损伤反应蛋白)的水平。此外,由于检测到裂解的 PARP,在两种细胞系中都证实了细胞凋亡。该复合物对小牛胸腺 DNA (CT-DNA) 和牛血清白蛋白 (BSA) 均表现出良好的结合亲和力,并且它们相互作用的顺序与其细胞毒性作用一致。还使用 CT-DNA 和 BSA 靶标对复合物进行了分子模拟。
更新日期:2023-12-27
中文翻译:

基于氨基喹啉的 Re(I) 三羰基复合物:深入了解其抗增殖活性和作用机制
为了开发新的有效抗癌药物,合成了两种希夫碱铼(I)三羰基络合物,其中含有普遍存在的氨基喹啉支架。氨基喹啉配体和 Re(I) 复合物在 48 小时的孵育期内均表现出足够的稳定性。此外,还针对激素依赖性 MCF-7 和激素非依赖性三阴性 MDA-MB-231 乳腺癌细胞系评估了前体配体和铼 (I) 配合物的细胞毒活性。 [Re(CO)Cl]实体的包含显着增强了氨基喹啉席夫碱配体对测试的癌细胞系的细胞毒性。值得注意的是,与亚氨基吡啶基实体相比,席夫碱亚氨基喹啉基实体的掺入显着增强了 Re(I) 复合物的细胞毒活性。值得注意的是,喹啉基取代的复合物对乳腺癌细胞系的活性比顺铂高出三倍,支撑了喹啉药效团在合理药物设计中的重要性。此外,与非致瘤性 FG-0 细胞相比,最活跃的 Re(I) 复合物对乳腺癌细胞表现出更好的选择性。蛋白质印迹显示,复合物增加了 γH2AX(一种关键的 DNA 损伤反应蛋白)的水平。此外,由于检测到裂解的 PARP,在两种细胞系中都证实了细胞凋亡。该复合物对小牛胸腺 DNA (CT-DNA) 和牛血清白蛋白 (BSA) 均表现出良好的结合亲和力,并且它们相互作用的顺序与其细胞毒性作用一致。还使用 CT-DNA 和 BSA 靶标对复合物进行了分子模拟。































 京公网安备 11010802027423号
京公网安备 11010802027423号