当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tropospheric Halogen Chemistry: Sources, Cycling, and Impacts
Chemical Reviews ( IF 51.4 ) Pub Date : 2015-03-12 00:00:00 , DOI: 10.1021/cr5006638 William R. Simpson 1 , Steven S. Brown 2 , Alfonso Saiz-Lopez 3 , Joel A. Thornton 4 , Roland von Glasow 5
Chemical Reviews ( IF 51.4 ) Pub Date : 2015-03-12 00:00:00 , DOI: 10.1021/cr5006638 William R. Simpson 1 , Steven S. Brown 2 , Alfonso Saiz-Lopez 3 , Joel A. Thornton 4 , Roland von Glasow 5
Affiliation
|
This article is part of the 2015 Chemistry in Climate special issue.  William Simpson graduated from Swarthmore College with a B.A. in Chemistry and a Mathematics minor in 1988. He received his Ph.D. in Physical Chemistry from Stanford University in 1995. After two postdoctoral appointments, he joined the faculty at University of Alaska Fairbanks in 1997, where he currently is a Professor of Chemistry. His research group investigates atmospheric halogen and nitrogen chemistry using spectroscopic methods with a focus on the Arctic region and how the changing climate affects halogen activation.
William Simpson graduated from Swarthmore College with a B.A. in Chemistry and a Mathematics minor in 1988. He received his Ph.D. in Physical Chemistry from Stanford University in 1995. After two postdoctoral appointments, he joined the faculty at University of Alaska Fairbanks in 1997, where he currently is a Professor of Chemistry. His research group investigates atmospheric halogen and nitrogen chemistry using spectroscopic methods with a focus on the Arctic region and how the changing climate affects halogen activation.  Steven Brown received his B.A. in Chemistry from Dartmouth College in 1989. He then received a Ph. D. in physical chemistry at the University of Wisconsin-Madison and came to the National Oceanic and Atmospheric Administration (NOAA) Laboratories in Boulder, Colorado in 1997. He is currently a Research Chemist at NOAA and serves as an adjoint professor at the University of Colorado. He studies the chemistry and impacts of nitrogen oxides in the Earth’s atmosphere, with and emphasis on field measurements of tropospheric nitrogen oxides, particularly those that occur in the dark (nighttime chemistry). His other main research interest has been the development of high sensitivity optical instrumentation for laboratory and field studies of atmospheric trace gases and aerosol particles.
Steven Brown received his B.A. in Chemistry from Dartmouth College in 1989. He then received a Ph. D. in physical chemistry at the University of Wisconsin-Madison and came to the National Oceanic and Atmospheric Administration (NOAA) Laboratories in Boulder, Colorado in 1997. He is currently a Research Chemist at NOAA and serves as an adjoint professor at the University of Colorado. He studies the chemistry and impacts of nitrogen oxides in the Earth’s atmosphere, with and emphasis on field measurements of tropospheric nitrogen oxides, particularly those that occur in the dark (nighttime chemistry). His other main research interest has been the development of high sensitivity optical instrumentation for laboratory and field studies of atmospheric trace gases and aerosol particles.  Alfonso Saiz-Lopez studied Chemistry in Ciudad Real, Spain. In 2006, he received his Ph.D. degree in Atmospheric Physical Chemistry at the University of East Anglia, focused on absorption spectroscopy for atmospheric measurement and marine boundary layer halogen chemistry. After a brief postdoctoral stay at the University of Leeds, he was a NASA Postdoctoral Scholar at the Jet Propulsion Laboratory and Research Associate at the Harvard-Smithsonian Center for Astrophysics. Since 2009, he is a Senior Research Scientist at the Spanish National Research Council (CSIC) and an Affiliate Scientist at the NCAR. The work of his group focuses on atmospheric halogen chemistry and its effect on climate.
Alfonso Saiz-Lopez studied Chemistry in Ciudad Real, Spain. In 2006, he received his Ph.D. degree in Atmospheric Physical Chemistry at the University of East Anglia, focused on absorption spectroscopy for atmospheric measurement and marine boundary layer halogen chemistry. After a brief postdoctoral stay at the University of Leeds, he was a NASA Postdoctoral Scholar at the Jet Propulsion Laboratory and Research Associate at the Harvard-Smithsonian Center for Astrophysics. Since 2009, he is a Senior Research Scientist at the Spanish National Research Council (CSIC) and an Affiliate Scientist at the NCAR. The work of his group focuses on atmospheric halogen chemistry and its effect on climate.  Joel Thornton obtained his B.A. in Chemistry from Dartmouth College in 1996. He obtained his Ph.D. in Chemistry from University of California, Berkeley in 2002. Following a postdoctoral appointment at University of Toronto, he entered the faculty at the University of Washington in 2004. He currently is an Associate Professor in the Department of Atmospheric Sciences at University of Washington, with an affiliate appointment in Chemistry. Research in his group utilizes state-of-the-art analytical techniques based on mass spectrometry and spectroscopy to study the physical chemistry of atmospheric phenomena, such as halogen chemistry, pollution chemistry, and aerosol particle formation/modification.
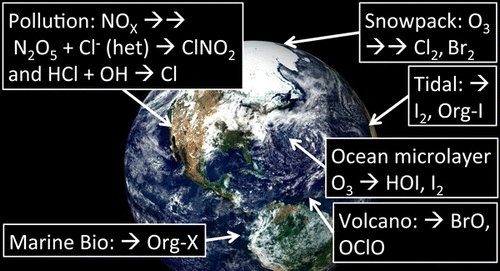
Joel Thornton obtained his B.A. in Chemistry from Dartmouth College in 1996. He obtained his Ph.D. in Chemistry from University of California, Berkeley in 2002. Following a postdoctoral appointment at University of Toronto, he entered the faculty at the University of Washington in 2004. He currently is an Associate Professor in the Department of Atmospheric Sciences at University of Washington, with an affiliate appointment in Chemistry. Research in his group utilizes state-of-the-art analytical techniques based on mass spectrometry and spectroscopy to study the physical chemistry of atmospheric phenomena, such as halogen chemistry, pollution chemistry, and aerosol particle formation/modification.  Roland von Glasow studied atmospheric physics at the University of Mainz, Germany; he then completed his Ph.D. on atmospheric chemistry at the Max-Planck-Institute (MPI) in Mainz, Germany, in 2001. He continued research as a postdoctoral fellow at MPI and then at the Scripps Institution of Oceanography, San Diego. He led a research group at the University of Heidelberg, Germany, for three years before moving to the University of East Anglia, U.K., in 2007, where he was promoted to Professor in 2012. He and his group develop and apply numerical models to study chemical and physical processes in the troposphere with a focus on reactive halogen chemistry. Regions of this investigation include the marine boundary layer, the polar regions, volcanic plumes, and the free troposphere. Figure 1. Simplified reaction diagram for halogen atoms, represented as “X” in this diagram, key chemical reaction pathways. Note that many species on this diagram are radicals, but for simplicity, only organic radicals and organic peroxy radicals, denoted by R• and RO2•, are explicitly shown with an unpaired electron. Figure 2. Primary sources of reactive halogen species or their precursor reservoir species overlain on a MODIS image of Earth. Background image produced by the MODIS Land Group, NASA Goddard Space Flight Center, Visible Earth Project, NASA. Only the last name of the first author for the reference is listed for space considerations. Northern and Southern hemisphere data are designated by NH and SH, respectively. As discussed below, tidal and open ocean areas differ in iodine emissions, so the table separates these locations. These mixing ratios represent selected values from recent literature and are not a comprehensive listing but are meant to roughly indicate the range of levels in these regions. The reviews of Saiz-Lopez and von Glasow(30) and Saiz-Lopez and co-workers(30) present comprehensive lists of observations published before 2012, and recent observations appear in this review and in this table. More recent observations at coastal and continetal sites are described in section 3.3.1. Figure 3. Br2 production during a snow chamber experiments on 27 March 2012. Tundra snow is exposed to ambient radiation and varying ozone levels, and produced Br2 is monitored. Reprinted with permission from Pratt and co-workers (2013).(43) Copyright 2013 Nature Publishing Group. Figure 4. Evolution of dihalogen gases from laboratory experiments simulating polar halogen activation by irradiating salt-doped ice particles with or without coexposure to ozone gas. Panel a shows dihalogens (Br2 in red, Cl2 in black, and BrCl in green. Panel b shows ozone, and yellow areas indicate times when the sample was irradiated. The dotted shading visible on top of the yellow indicates when the ozone generator was switched on (dotted = ozone on, no dots = ozone off). Reprinted with permission from Wren and co-workers (2013).(110) Copyright Wren and co-workers 2013. CC Attribution 3.0 License. Figure 5. Simulated column amounts of BrO in the atmosphere versus time from the 1-D model of Toyota and co-workers (2014).(143) The panels show different assumptions for turbulence in the atmospheric boundary layer based upon windspeed, U2, and various values of the Brunt–Väisälä frequency, N. Note that the BrO column scale on each panel is different, and the higher windspeeds show much higher VCDs. Reprinted with permission from Toyota and co-workers (2014).(143) Copyright Toyota and co-workers 2014. CC Attribution 3.0 License. Figure 6. Marine boundary layer iodine monoxide (IO) observations from ship cruise and coastal station observations in pmol/mol. Reprinted with permission from Prados-Roman and co-workers (2015).(194) Copyright Prados-Roman and co-workers 2015. CC Attribution 3.0 License. Figure 7. Nightime observations of NOx polluted air containing N2O5 and produced reactive halogen precursor, nitryl chloride, ClNO2, measured at the Scripps Institution of Oceanography Pier. Reprinted with permission from Kim and co-workers (2014).(212) Copyright 2014 National Academy of Sciences. Figure 8. Conversion efficiency for production of ClNO2 from N2O5 from laboratory measurements along with models. Panel b shows ranges of particulate chloride composition from the TexAQS-GoMACCS 2006 field campaign. Reprinted with permission from Roberts and co-workers (2009).(217) Copyright 2009 American Geophysical Union. Figure 9. Mean of nightly 1 h maximum ClNO2 in January without the heterogeneous ClNO2 production (pptv = pmol/mol), (b) mean of nightly 1 h maximum ClNO2 in June without the heterogeneous ClNO2 production (pptv = pmol/mol), (c) mean of nightly 1 h maximum ClNO2 in January with the heterogeneous ClNO2 production (ppbv = nmol/mol), and (d) mean of nightly 1 h maximum ClNO2 in June with the heterogeneous ClNO2 production (ppbv = nmol/mol). Reprinted with permission from Sarwar and co-workers (2014).(222) Copyright 2014 American Geophysical Union. Figure 10. Seasonal variation of mean annual tropospheric BrO simulated by GEOS-Chem along with GOME-2 observations and p-TOMCAT model simulations. The effect of removing the heterogeneous reaction of HBr + HOBr is demonstrated in the GEOS-Chem simulations. Reprinted with permission from Parrella and co-workers (2014).(258) Copyright Parrella and co-workers 2014. CC Attribution 3.0 License. Figure 11. Modeled anthropogenic influence on oceanic iodine source as a percentage change from preindustrial times to current conditions. Reprinted with permission from Prados-Roman and co-workers.(262) Copyright Prados-Roman and co-workers 2015. CC Attribution 3.0 License. The authors declare no competing financial interest. The authors thank their respective institutions and funding agencies (National Science Foundation (US), National Aeronautics and Space Administration (US), National Oceanic and Atmospheric Administration (US), Natural Environment Research Council (UK), The Deutsche Forschungsgemeinschaft (Germany), the Spanish National Research Council, CSIC (Spain), and the European Research Council) for support in carrying out this research and review effort. We also thank an anonymous reviewer for constructive comments that improved this article. This article references 266 other publications.
Roland von Glasow studied atmospheric physics at the University of Mainz, Germany; he then completed his Ph.D. on atmospheric chemistry at the Max-Planck-Institute (MPI) in Mainz, Germany, in 2001. He continued research as a postdoctoral fellow at MPI and then at the Scripps Institution of Oceanography, San Diego. He led a research group at the University of Heidelberg, Germany, for three years before moving to the University of East Anglia, U.K., in 2007, where he was promoted to Professor in 2012. He and his group develop and apply numerical models to study chemical and physical processes in the troposphere with a focus on reactive halogen chemistry. Regions of this investigation include the marine boundary layer, the polar regions, volcanic plumes, and the free troposphere. Figure 1. Simplified reaction diagram for halogen atoms, represented as “X” in this diagram, key chemical reaction pathways. Note that many species on this diagram are radicals, but for simplicity, only organic radicals and organic peroxy radicals, denoted by R• and RO2•, are explicitly shown with an unpaired electron. Figure 2. Primary sources of reactive halogen species or their precursor reservoir species overlain on a MODIS image of Earth. Background image produced by the MODIS Land Group, NASA Goddard Space Flight Center, Visible Earth Project, NASA. Only the last name of the first author for the reference is listed for space considerations. Northern and Southern hemisphere data are designated by NH and SH, respectively. As discussed below, tidal and open ocean areas differ in iodine emissions, so the table separates these locations. These mixing ratios represent selected values from recent literature and are not a comprehensive listing but are meant to roughly indicate the range of levels in these regions. The reviews of Saiz-Lopez and von Glasow(30) and Saiz-Lopez and co-workers(30) present comprehensive lists of observations published before 2012, and recent observations appear in this review and in this table. More recent observations at coastal and continetal sites are described in section 3.3.1. Figure 3. Br2 production during a snow chamber experiments on 27 March 2012. Tundra snow is exposed to ambient radiation and varying ozone levels, and produced Br2 is monitored. Reprinted with permission from Pratt and co-workers (2013).(43) Copyright 2013 Nature Publishing Group. Figure 4. Evolution of dihalogen gases from laboratory experiments simulating polar halogen activation by irradiating salt-doped ice particles with or without coexposure to ozone gas. Panel a shows dihalogens (Br2 in red, Cl2 in black, and BrCl in green. Panel b shows ozone, and yellow areas indicate times when the sample was irradiated. The dotted shading visible on top of the yellow indicates when the ozone generator was switched on (dotted = ozone on, no dots = ozone off). Reprinted with permission from Wren and co-workers (2013).(110) Copyright Wren and co-workers 2013. CC Attribution 3.0 License. Figure 5. Simulated column amounts of BrO in the atmosphere versus time from the 1-D model of Toyota and co-workers (2014).(143) The panels show different assumptions for turbulence in the atmospheric boundary layer based upon windspeed, U2, and various values of the Brunt–Väisälä frequency, N. Note that the BrO column scale on each panel is different, and the higher windspeeds show much higher VCDs. Reprinted with permission from Toyota and co-workers (2014).(143) Copyright Toyota and co-workers 2014. CC Attribution 3.0 License. Figure 6. Marine boundary layer iodine monoxide (IO) observations from ship cruise and coastal station observations in pmol/mol. Reprinted with permission from Prados-Roman and co-workers (2015).(194) Copyright Prados-Roman and co-workers 2015. CC Attribution 3.0 License. Figure 7. Nightime observations of NOx polluted air containing N2O5 and produced reactive halogen precursor, nitryl chloride, ClNO2, measured at the Scripps Institution of Oceanography Pier. Reprinted with permission from Kim and co-workers (2014).(212) Copyright 2014 National Academy of Sciences. Figure 8. Conversion efficiency for production of ClNO2 from N2O5 from laboratory measurements along with models. Panel b shows ranges of particulate chloride composition from the TexAQS-GoMACCS 2006 field campaign. Reprinted with permission from Roberts and co-workers (2009).(217) Copyright 2009 American Geophysical Union. Figure 9. Mean of nightly 1 h maximum ClNO2 in January without the heterogeneous ClNO2 production (pptv = pmol/mol), (b) mean of nightly 1 h maximum ClNO2 in June without the heterogeneous ClNO2 production (pptv = pmol/mol), (c) mean of nightly 1 h maximum ClNO2 in January with the heterogeneous ClNO2 production (ppbv = nmol/mol), and (d) mean of nightly 1 h maximum ClNO2 in June with the heterogeneous ClNO2 production (ppbv = nmol/mol). Reprinted with permission from Sarwar and co-workers (2014).(222) Copyright 2014 American Geophysical Union. Figure 10. Seasonal variation of mean annual tropospheric BrO simulated by GEOS-Chem along with GOME-2 observations and p-TOMCAT model simulations. The effect of removing the heterogeneous reaction of HBr + HOBr is demonstrated in the GEOS-Chem simulations. Reprinted with permission from Parrella and co-workers (2014).(258) Copyright Parrella and co-workers 2014. CC Attribution 3.0 License. Figure 11. Modeled anthropogenic influence on oceanic iodine source as a percentage change from preindustrial times to current conditions. Reprinted with permission from Prados-Roman and co-workers.(262) Copyright Prados-Roman and co-workers 2015. CC Attribution 3.0 License. The authors declare no competing financial interest. The authors thank their respective institutions and funding agencies (National Science Foundation (US), National Aeronautics and Space Administration (US), National Oceanic and Atmospheric Administration (US), Natural Environment Research Council (UK), The Deutsche Forschungsgemeinschaft (Germany), the Spanish National Research Council, CSIC (Spain), and the European Research Council) for support in carrying out this research and review effort. We also thank an anonymous reviewer for constructive comments that improved this article. This article references 266 other publications.
中文翻译:

对流层卤素化学:来源,循环和影响
本文是2015年气候化学 特刊。 威廉·辛普森(William Simpson)于1988年毕业于斯沃斯莫尔学院(Swarthmore College),获得了化学学士学位和数学专业的辅修学位。他于1995年在斯坦福大学获得物理化学博士学位。在两次博士后任命后,他于1997年加入阿拉斯加费尔班克斯大学,现任化学教授。他的研究小组使用光谱方法研究了大气中的卤素和氮化学,重点是北极地区以及不断变化的气候如何影响卤素的活化。
威廉·辛普森(William Simpson)于1988年毕业于斯沃斯莫尔学院(Swarthmore College),获得了化学学士学位和数学专业的辅修学位。他于1995年在斯坦福大学获得物理化学博士学位。在两次博士后任命后,他于1997年加入阿拉斯加费尔班克斯大学,现任化学教授。他的研究小组使用光谱方法研究了大气中的卤素和氮化学,重点是北极地区以及不断变化的气候如何影响卤素的活化。 史蒂文·布朗(Steven Brown)于1989年从达特茅斯学院(Dartmouth College)获得化学学士学位。随后,他在威斯康星大学麦迪逊分校获得物理化学博士学位,并于1997年进入科罗拉多州博尔德的国家海洋与大气管理局(NOAA)实验室。他目前是NOAA的研究化学家,并担任科罗拉多大学的副教授。他研究了地球大气中氮氧化物的化学性质和影响,并重点研究了对流层氮氧化物的现场测量,尤其是在黑暗中发生的那些(夜间化学反应)。他的另一个主要研究兴趣是开发用于大气和痕量气体和气溶胶颗粒的实验室和现场研究的高灵敏度光学仪器。
史蒂文·布朗(Steven Brown)于1989年从达特茅斯学院(Dartmouth College)获得化学学士学位。随后,他在威斯康星大学麦迪逊分校获得物理化学博士学位,并于1997年进入科罗拉多州博尔德的国家海洋与大气管理局(NOAA)实验室。他目前是NOAA的研究化学家,并担任科罗拉多大学的副教授。他研究了地球大气中氮氧化物的化学性质和影响,并重点研究了对流层氮氧化物的现场测量,尤其是在黑暗中发生的那些(夜间化学反应)。他的另一个主要研究兴趣是开发用于大气和痕量气体和气溶胶颗粒的实验室和现场研究的高灵敏度光学仪器。 阿方索·萨伊兹·洛佩兹(Alfonso Saiz-Lopez)在西班牙雷阿尔城学习化学。2006年,他获得了博士学位。在东英吉利大学获得大气物理化学博士学位,重点是用于大气测量和海洋边界层卤素化学的吸收光谱学。在利兹大学短暂的博士后逗留之后,他是美国喷气机推进实验室的NASA博士后学者,以及哈佛-史密森尼天体物理学中心的研究助理。自2009年以来,他是西班牙国家研究委员会(CSIC)的高级研究科学家和NCAR的附属科学家。他的小组的工作重点是大气中的卤素化学及其对气候的影响。
阿方索·萨伊兹·洛佩兹(Alfonso Saiz-Lopez)在西班牙雷阿尔城学习化学。2006年,他获得了博士学位。在东英吉利大学获得大气物理化学博士学位,重点是用于大气测量和海洋边界层卤素化学的吸收光谱学。在利兹大学短暂的博士后逗留之后,他是美国喷气机推进实验室的NASA博士后学者,以及哈佛-史密森尼天体物理学中心的研究助理。自2009年以来,他是西班牙国家研究委员会(CSIC)的高级研究科学家和NCAR的附属科学家。他的小组的工作重点是大气中的卤素化学及其对气候的影响。 乔尔·桑顿(Joel Thornton)于1996年从达特茅斯学院(Dartmouth College)获得化学学士学位。他于2002年在加州大学伯克利分校获得化学博士学位。在多伦多大学获得博士学位后,他于2004年进入华盛顿大学任教。他目前是华盛顿大学大气科学系的副教授,化学方面的会员任命。他的研究小组利用基于质谱和光谱学的最新分析技术来研究大气现象的物理化学,例如卤素化学,污染化学和气溶胶颗粒的形成/修饰。
乔尔·桑顿(Joel Thornton)于1996年从达特茅斯学院(Dartmouth College)获得化学学士学位。他于2002年在加州大学伯克利分校获得化学博士学位。在多伦多大学获得博士学位后,他于2004年进入华盛顿大学任教。他目前是华盛顿大学大气科学系的副教授,化学方面的会员任命。他的研究小组利用基于质谱和光谱学的最新分析技术来研究大气现象的物理化学,例如卤素化学,污染化学和气溶胶颗粒的形成/修饰。 罗兰·冯·格拉索(Roland von Glasow)在德国美因兹大学学习了大气物理学。然后,他完成了博士学位。2001年,在德国美因兹的马克斯-普朗克研究所(MPI)从事大气化学研究。他继续在MPI以及圣地亚哥的斯克里普斯海洋学研究所担任博士后研究员。在2007年移居英国东安格利亚大学之前,他领导了德国海德堡大学的一个研究小组三年,于2012年被提升为教授。他和他的小组开发并应用了数值模型进行研究对流层的化学和物理过程,重点是反应性卤素化学。该调查的区域包括海洋边界层,极地区域,火山羽和自由对流层。图1.卤素原子的简化反应图,在此图中以“ X”表示的是关键的化学反应路径。请注意,此图上的许多物种都是自由基,但为简单起见,只有有机自由基和有机过氧自由基,用R表示•和RO 2 •明确地显示未成对电子。图2.地球上的MODIS图像上覆盖了活性卤素物种或其前驱储层物种的主要来源。由美国国家航空航天局戈达德太空飞行中心,美国国家航空航天局可见地球项目MODIS土地小组制作的背景图片。出于篇幅考虑,仅列出了第一作者的姓氏作为参考。北半球和南半球的数据分别由NH和SH表示。如下所述,潮汐和公海区域的碘排放量有所不同,因此该表将这些位置分开。这些混合比代表了从最近文献中选择的值,并不是全面的清单,而是用来粗略地指出这些区域的含量范围。Saiz-Lopez和von Glasow(30)以及Saiz-Lopez和同事(30)的评论提供了2012年之前发表的观察的全面列表,最近的观察出现在此评论和此表中。在3.3.1节中介绍了近海和沿海地区的最新观测资料。图3. Br在2012年3月27日的雪室实验中产生了2的产量。苔原雪暴露于环境辐射和变化的臭氧水平下,并监测所产生的Br 2。未经Pratt和同事的许可转载(2013)。(43)版权所有2013 Nature Publishing Group。图4.来自实验室实验的二卤气体的演变,该实验通过在有或没有与臭氧气体共暴露的情况下照射掺盐的冰粒来模拟极性卤素的活化。小图a显示了二卤素(红色的Br 2,Cl 2黑色为BrCl,绿色为BrCl。面板b显示臭氧,黄色区域表示样品被辐照的时间。黄色上方可见的虚线阴影表示打开臭氧发生器的时间(虚线=臭氧打开,无点=臭氧关闭)。经Wren和同事的许可转载(2013)。(110)Wren和同事的版权2013。CC署名3.0许可。图5.丰田及其同事的一维模型(2014)中大气中BrO随时间变化的模拟柱量。(143)面板显示了基于风速U 2的大气边界层湍流的不同假设。,以及Brunt–Väisälä频率的各种值N。请注意,每个面板上的BrO列刻度不同,并且更高的风速显示更高的VCD。经丰田及其同事许可(2014年)转载。(143)丰田及其同事2014年版权所有。CCAttribution 3.0 License。图6.船舶巡游和海岸站观测中的海洋边界层一氧化碘(IO)观测值,以pmol / mol为单位。经Prados-Roman及其同事许可(2015)。(194)版权所有Prados-Roman及其同事2015。CCAttribution 3.0 License。图7.含有N 2 O 5和产生的活性卤素前体,硝酰氯,ClNO 2的NO x污染空气的夜间观察,由斯克里普斯海洋学码头学会(Scripps Institution of Oceanography Pier)测量。未经Kim和其同事许可转载(2014)。(212)2014年美国国家科学院版权所有。图8.实验室测量和模型从N 2 O 5产生ClNO 2的转化效率。面板b显示了TexAQS-GoMACCS 2006野外活动中氯化物颗粒组成的范围。经罗伯茨(Roberts)和同事的许可转载(2009)。(217)版权所有©美国地球物理联盟,2009年。图9.一月份没有异质ClNO 2产生的最大夜间ClNO 2的平均值(pptv = pmol / mol),(b)六月没有异质ClNO的最大夜间ClNO 2的平均值2生产(PPTV =皮摩尔/摩尔),(C)的平均的夜间1个小时最大ClNO 2月份与异质ClNO 2生产(ppbv的=纳摩尔/摩尔),和(d)平均夜间1个小时最大ClNO 2在异构ClNO 2的六月产物(ppbv = nmol / mol)。经Sarwar及其同事许可转载(2014)。(222)版权所有2014 American Earthphysical Union。图10.由GEOS-Chem模拟的年平均对流层BrO的季节变化以及GOME-2观测值和p-TOMCAT模型模拟。GEOS-Chem模拟显示了去除HBr + HOBr异质反应的效果。经Parrella及其同事许可(2014)重印。(258)Parrella及其同事2014版权所有。CCAttribution 3.0 License。图11.模拟的人为对海洋碘源的影响(从工业化前时期到当前状况的百分比变化)。经Prados-Roman及其同事许可转载。(262)版权所有Prados-Roman及其同事2015。CCAttribution 3.0许可证。作者宣称没有竞争性的经济利益。作者感谢其各自的机构和资助机构(国家科学基金会(美国),国家航空航天局(美国),国家海洋和大气管理局(美国),自然环境研究委员会(英国),德国科学基金会)(德国),西班牙国家研究委员会,CSIC(西班牙)和欧洲研究委员会)为开展这项研究和审查工作提供的支持。我们也感谢一位匿名审稿人对本文的建设性评论做出了改进。本文引用了266个其他出版物。自然环境研究委员会(英国),德国科学基金会(德国),西班牙国家研究委员会,CSIC(西班牙)和欧洲研究委员会)为开展这项研究和评估工作提供了支持。我们也感谢一位匿名审稿人对本文的建设性评论做出了改进。本文引用了266个其他出版物。自然环境研究委员会(英国),德国科学基金会(德国),西班牙国家研究委员会,CSIC(西班牙)和欧洲研究委员会)为开展这项研究和评估工作提供了支持。我们也感谢一位匿名审稿人对本文的建设性评论做出了改进。本文引用了266个其他出版物。
罗兰·冯·格拉索(Roland von Glasow)在德国美因兹大学学习了大气物理学。然后,他完成了博士学位。2001年,在德国美因兹的马克斯-普朗克研究所(MPI)从事大气化学研究。他继续在MPI以及圣地亚哥的斯克里普斯海洋学研究所担任博士后研究员。在2007年移居英国东安格利亚大学之前,他领导了德国海德堡大学的一个研究小组三年,于2012年被提升为教授。他和他的小组开发并应用了数值模型进行研究对流层的化学和物理过程,重点是反应性卤素化学。该调查的区域包括海洋边界层,极地区域,火山羽和自由对流层。图1.卤素原子的简化反应图,在此图中以“ X”表示的是关键的化学反应路径。请注意,此图上的许多物种都是自由基,但为简单起见,只有有机自由基和有机过氧自由基,用R表示•和RO 2 •明确地显示未成对电子。图2.地球上的MODIS图像上覆盖了活性卤素物种或其前驱储层物种的主要来源。由美国国家航空航天局戈达德太空飞行中心,美国国家航空航天局可见地球项目MODIS土地小组制作的背景图片。出于篇幅考虑,仅列出了第一作者的姓氏作为参考。北半球和南半球的数据分别由NH和SH表示。如下所述,潮汐和公海区域的碘排放量有所不同,因此该表将这些位置分开。这些混合比代表了从最近文献中选择的值,并不是全面的清单,而是用来粗略地指出这些区域的含量范围。Saiz-Lopez和von Glasow(30)以及Saiz-Lopez和同事(30)的评论提供了2012年之前发表的观察的全面列表,最近的观察出现在此评论和此表中。在3.3.1节中介绍了近海和沿海地区的最新观测资料。图3. Br在2012年3月27日的雪室实验中产生了2的产量。苔原雪暴露于环境辐射和变化的臭氧水平下,并监测所产生的Br 2。未经Pratt和同事的许可转载(2013)。(43)版权所有2013 Nature Publishing Group。图4.来自实验室实验的二卤气体的演变,该实验通过在有或没有与臭氧气体共暴露的情况下照射掺盐的冰粒来模拟极性卤素的活化。小图a显示了二卤素(红色的Br 2,Cl 2黑色为BrCl,绿色为BrCl。面板b显示臭氧,黄色区域表示样品被辐照的时间。黄色上方可见的虚线阴影表示打开臭氧发生器的时间(虚线=臭氧打开,无点=臭氧关闭)。经Wren和同事的许可转载(2013)。(110)Wren和同事的版权2013。CC署名3.0许可。图5.丰田及其同事的一维模型(2014)中大气中BrO随时间变化的模拟柱量。(143)面板显示了基于风速U 2的大气边界层湍流的不同假设。,以及Brunt–Väisälä频率的各种值N。请注意,每个面板上的BrO列刻度不同,并且更高的风速显示更高的VCD。经丰田及其同事许可(2014年)转载。(143)丰田及其同事2014年版权所有。CCAttribution 3.0 License。图6.船舶巡游和海岸站观测中的海洋边界层一氧化碘(IO)观测值,以pmol / mol为单位。经Prados-Roman及其同事许可(2015)。(194)版权所有Prados-Roman及其同事2015。CCAttribution 3.0 License。图7.含有N 2 O 5和产生的活性卤素前体,硝酰氯,ClNO 2的NO x污染空气的夜间观察,由斯克里普斯海洋学码头学会(Scripps Institution of Oceanography Pier)测量。未经Kim和其同事许可转载(2014)。(212)2014年美国国家科学院版权所有。图8.实验室测量和模型从N 2 O 5产生ClNO 2的转化效率。面板b显示了TexAQS-GoMACCS 2006野外活动中氯化物颗粒组成的范围。经罗伯茨(Roberts)和同事的许可转载(2009)。(217)版权所有©美国地球物理联盟,2009年。图9.一月份没有异质ClNO 2产生的最大夜间ClNO 2的平均值(pptv = pmol / mol),(b)六月没有异质ClNO的最大夜间ClNO 2的平均值2生产(PPTV =皮摩尔/摩尔),(C)的平均的夜间1个小时最大ClNO 2月份与异质ClNO 2生产(ppbv的=纳摩尔/摩尔),和(d)平均夜间1个小时最大ClNO 2在异构ClNO 2的六月产物(ppbv = nmol / mol)。经Sarwar及其同事许可转载(2014)。(222)版权所有2014 American Earthphysical Union。图10.由GEOS-Chem模拟的年平均对流层BrO的季节变化以及GOME-2观测值和p-TOMCAT模型模拟。GEOS-Chem模拟显示了去除HBr + HOBr异质反应的效果。经Parrella及其同事许可(2014)重印。(258)Parrella及其同事2014版权所有。CCAttribution 3.0 License。图11.模拟的人为对海洋碘源的影响(从工业化前时期到当前状况的百分比变化)。经Prados-Roman及其同事许可转载。(262)版权所有Prados-Roman及其同事2015。CCAttribution 3.0许可证。作者宣称没有竞争性的经济利益。作者感谢其各自的机构和资助机构(国家科学基金会(美国),国家航空航天局(美国),国家海洋和大气管理局(美国),自然环境研究委员会(英国),德国科学基金会)(德国),西班牙国家研究委员会,CSIC(西班牙)和欧洲研究委员会)为开展这项研究和审查工作提供的支持。我们也感谢一位匿名审稿人对本文的建设性评论做出了改进。本文引用了266个其他出版物。自然环境研究委员会(英国),德国科学基金会(德国),西班牙国家研究委员会,CSIC(西班牙)和欧洲研究委员会)为开展这项研究和评估工作提供了支持。我们也感谢一位匿名审稿人对本文的建设性评论做出了改进。本文引用了266个其他出版物。自然环境研究委员会(英国),德国科学基金会(德国),西班牙国家研究委员会,CSIC(西班牙)和欧洲研究委员会)为开展这项研究和评估工作提供了支持。我们也感谢一位匿名审稿人对本文的建设性评论做出了改进。本文引用了266个其他出版物。
更新日期:2015-03-12
 William Simpson graduated from Swarthmore College with a B.A. in Chemistry and a Mathematics minor in 1988. He received his Ph.D. in Physical Chemistry from Stanford University in 1995. After two postdoctoral appointments, he joined the faculty at University of Alaska Fairbanks in 1997, where he currently is a Professor of Chemistry. His research group investigates atmospheric halogen and nitrogen chemistry using spectroscopic methods with a focus on the Arctic region and how the changing climate affects halogen activation.
William Simpson graduated from Swarthmore College with a B.A. in Chemistry and a Mathematics minor in 1988. He received his Ph.D. in Physical Chemistry from Stanford University in 1995. After two postdoctoral appointments, he joined the faculty at University of Alaska Fairbanks in 1997, where he currently is a Professor of Chemistry. His research group investigates atmospheric halogen and nitrogen chemistry using spectroscopic methods with a focus on the Arctic region and how the changing climate affects halogen activation.  Steven Brown received his B.A. in Chemistry from Dartmouth College in 1989. He then received a Ph. D. in physical chemistry at the University of Wisconsin-Madison and came to the National Oceanic and Atmospheric Administration (NOAA) Laboratories in Boulder, Colorado in 1997. He is currently a Research Chemist at NOAA and serves as an adjoint professor at the University of Colorado. He studies the chemistry and impacts of nitrogen oxides in the Earth’s atmosphere, with and emphasis on field measurements of tropospheric nitrogen oxides, particularly those that occur in the dark (nighttime chemistry). His other main research interest has been the development of high sensitivity optical instrumentation for laboratory and field studies of atmospheric trace gases and aerosol particles.
Steven Brown received his B.A. in Chemistry from Dartmouth College in 1989. He then received a Ph. D. in physical chemistry at the University of Wisconsin-Madison and came to the National Oceanic and Atmospheric Administration (NOAA) Laboratories in Boulder, Colorado in 1997. He is currently a Research Chemist at NOAA and serves as an adjoint professor at the University of Colorado. He studies the chemistry and impacts of nitrogen oxides in the Earth’s atmosphere, with and emphasis on field measurements of tropospheric nitrogen oxides, particularly those that occur in the dark (nighttime chemistry). His other main research interest has been the development of high sensitivity optical instrumentation for laboratory and field studies of atmospheric trace gases and aerosol particles.  Alfonso Saiz-Lopez studied Chemistry in Ciudad Real, Spain. In 2006, he received his Ph.D. degree in Atmospheric Physical Chemistry at the University of East Anglia, focused on absorption spectroscopy for atmospheric measurement and marine boundary layer halogen chemistry. After a brief postdoctoral stay at the University of Leeds, he was a NASA Postdoctoral Scholar at the Jet Propulsion Laboratory and Research Associate at the Harvard-Smithsonian Center for Astrophysics. Since 2009, he is a Senior Research Scientist at the Spanish National Research Council (CSIC) and an Affiliate Scientist at the NCAR. The work of his group focuses on atmospheric halogen chemistry and its effect on climate.
Alfonso Saiz-Lopez studied Chemistry in Ciudad Real, Spain. In 2006, he received his Ph.D. degree in Atmospheric Physical Chemistry at the University of East Anglia, focused on absorption spectroscopy for atmospheric measurement and marine boundary layer halogen chemistry. After a brief postdoctoral stay at the University of Leeds, he was a NASA Postdoctoral Scholar at the Jet Propulsion Laboratory and Research Associate at the Harvard-Smithsonian Center for Astrophysics. Since 2009, he is a Senior Research Scientist at the Spanish National Research Council (CSIC) and an Affiliate Scientist at the NCAR. The work of his group focuses on atmospheric halogen chemistry and its effect on climate.  Joel Thornton obtained his B.A. in Chemistry from Dartmouth College in 1996. He obtained his Ph.D. in Chemistry from University of California, Berkeley in 2002. Following a postdoctoral appointment at University of Toronto, he entered the faculty at the University of Washington in 2004. He currently is an Associate Professor in the Department of Atmospheric Sciences at University of Washington, with an affiliate appointment in Chemistry. Research in his group utilizes state-of-the-art analytical techniques based on mass spectrometry and spectroscopy to study the physical chemistry of atmospheric phenomena, such as halogen chemistry, pollution chemistry, and aerosol particle formation/modification.
Joel Thornton obtained his B.A. in Chemistry from Dartmouth College in 1996. He obtained his Ph.D. in Chemistry from University of California, Berkeley in 2002. Following a postdoctoral appointment at University of Toronto, he entered the faculty at the University of Washington in 2004. He currently is an Associate Professor in the Department of Atmospheric Sciences at University of Washington, with an affiliate appointment in Chemistry. Research in his group utilizes state-of-the-art analytical techniques based on mass spectrometry and spectroscopy to study the physical chemistry of atmospheric phenomena, such as halogen chemistry, pollution chemistry, and aerosol particle formation/modification.  Roland von Glasow studied atmospheric physics at the University of Mainz, Germany; he then completed his Ph.D. on atmospheric chemistry at the Max-Planck-Institute (MPI) in Mainz, Germany, in 2001. He continued research as a postdoctoral fellow at MPI and then at the Scripps Institution of Oceanography, San Diego. He led a research group at the University of Heidelberg, Germany, for three years before moving to the University of East Anglia, U.K., in 2007, where he was promoted to Professor in 2012. He and his group develop and apply numerical models to study chemical and physical processes in the troposphere with a focus on reactive halogen chemistry. Regions of this investigation include the marine boundary layer, the polar regions, volcanic plumes, and the free troposphere. Figure 1. Simplified reaction diagram for halogen atoms, represented as “X” in this diagram, key chemical reaction pathways. Note that many species on this diagram are radicals, but for simplicity, only organic radicals and organic peroxy radicals, denoted by R• and RO2•, are explicitly shown with an unpaired electron. Figure 2. Primary sources of reactive halogen species or their precursor reservoir species overlain on a MODIS image of Earth. Background image produced by the MODIS Land Group, NASA Goddard Space Flight Center, Visible Earth Project, NASA. Only the last name of the first author for the reference is listed for space considerations. Northern and Southern hemisphere data are designated by NH and SH, respectively. As discussed below, tidal and open ocean areas differ in iodine emissions, so the table separates these locations. These mixing ratios represent selected values from recent literature and are not a comprehensive listing but are meant to roughly indicate the range of levels in these regions. The reviews of Saiz-Lopez and von Glasow(30) and Saiz-Lopez and co-workers(30) present comprehensive lists of observations published before 2012, and recent observations appear in this review and in this table. More recent observations at coastal and continetal sites are described in section 3.3.1. Figure 3. Br2 production during a snow chamber experiments on 27 March 2012. Tundra snow is exposed to ambient radiation and varying ozone levels, and produced Br2 is monitored. Reprinted with permission from Pratt and co-workers (2013).(43) Copyright 2013 Nature Publishing Group. Figure 4. Evolution of dihalogen gases from laboratory experiments simulating polar halogen activation by irradiating salt-doped ice particles with or without coexposure to ozone gas. Panel a shows dihalogens (Br2 in red, Cl2 in black, and BrCl in green. Panel b shows ozone, and yellow areas indicate times when the sample was irradiated. The dotted shading visible on top of the yellow indicates when the ozone generator was switched on (dotted = ozone on, no dots = ozone off). Reprinted with permission from Wren and co-workers (2013).(110) Copyright Wren and co-workers 2013. CC Attribution 3.0 License. Figure 5. Simulated column amounts of BrO in the atmosphere versus time from the 1-D model of Toyota and co-workers (2014).(143) The panels show different assumptions for turbulence in the atmospheric boundary layer based upon windspeed, U2, and various values of the Brunt–Väisälä frequency, N. Note that the BrO column scale on each panel is different, and the higher windspeeds show much higher VCDs. Reprinted with permission from Toyota and co-workers (2014).(143) Copyright Toyota and co-workers 2014. CC Attribution 3.0 License. Figure 6. Marine boundary layer iodine monoxide (IO) observations from ship cruise and coastal station observations in pmol/mol. Reprinted with permission from Prados-Roman and co-workers (2015).(194) Copyright Prados-Roman and co-workers 2015. CC Attribution 3.0 License. Figure 7. Nightime observations of NOx polluted air containing N2O5 and produced reactive halogen precursor, nitryl chloride, ClNO2, measured at the Scripps Institution of Oceanography Pier. Reprinted with permission from Kim and co-workers (2014).(212) Copyright 2014 National Academy of Sciences. Figure 8. Conversion efficiency for production of ClNO2 from N2O5 from laboratory measurements along with models. Panel b shows ranges of particulate chloride composition from the TexAQS-GoMACCS 2006 field campaign. Reprinted with permission from Roberts and co-workers (2009).(217) Copyright 2009 American Geophysical Union. Figure 9. Mean of nightly 1 h maximum ClNO2 in January without the heterogeneous ClNO2 production (pptv = pmol/mol), (b) mean of nightly 1 h maximum ClNO2 in June without the heterogeneous ClNO2 production (pptv = pmol/mol), (c) mean of nightly 1 h maximum ClNO2 in January with the heterogeneous ClNO2 production (ppbv = nmol/mol), and (d) mean of nightly 1 h maximum ClNO2 in June with the heterogeneous ClNO2 production (ppbv = nmol/mol). Reprinted with permission from Sarwar and co-workers (2014).(222) Copyright 2014 American Geophysical Union. Figure 10. Seasonal variation of mean annual tropospheric BrO simulated by GEOS-Chem along with GOME-2 observations and p-TOMCAT model simulations. The effect of removing the heterogeneous reaction of HBr + HOBr is demonstrated in the GEOS-Chem simulations. Reprinted with permission from Parrella and co-workers (2014).(258) Copyright Parrella and co-workers 2014. CC Attribution 3.0 License. Figure 11. Modeled anthropogenic influence on oceanic iodine source as a percentage change from preindustrial times to current conditions. Reprinted with permission from Prados-Roman and co-workers.(262) Copyright Prados-Roman and co-workers 2015. CC Attribution 3.0 License. The authors declare no competing financial interest. The authors thank their respective institutions and funding agencies (National Science Foundation (US), National Aeronautics and Space Administration (US), National Oceanic and Atmospheric Administration (US), Natural Environment Research Council (UK), The Deutsche Forschungsgemeinschaft (Germany), the Spanish National Research Council, CSIC (Spain), and the European Research Council) for support in carrying out this research and review effort. We also thank an anonymous reviewer for constructive comments that improved this article. This article references 266 other publications.
Roland von Glasow studied atmospheric physics at the University of Mainz, Germany; he then completed his Ph.D. on atmospheric chemistry at the Max-Planck-Institute (MPI) in Mainz, Germany, in 2001. He continued research as a postdoctoral fellow at MPI and then at the Scripps Institution of Oceanography, San Diego. He led a research group at the University of Heidelberg, Germany, for three years before moving to the University of East Anglia, U.K., in 2007, where he was promoted to Professor in 2012. He and his group develop and apply numerical models to study chemical and physical processes in the troposphere with a focus on reactive halogen chemistry. Regions of this investigation include the marine boundary layer, the polar regions, volcanic plumes, and the free troposphere. Figure 1. Simplified reaction diagram for halogen atoms, represented as “X” in this diagram, key chemical reaction pathways. Note that many species on this diagram are radicals, but for simplicity, only organic radicals and organic peroxy radicals, denoted by R• and RO2•, are explicitly shown with an unpaired electron. Figure 2. Primary sources of reactive halogen species or their precursor reservoir species overlain on a MODIS image of Earth. Background image produced by the MODIS Land Group, NASA Goddard Space Flight Center, Visible Earth Project, NASA. Only the last name of the first author for the reference is listed for space considerations. Northern and Southern hemisphere data are designated by NH and SH, respectively. As discussed below, tidal and open ocean areas differ in iodine emissions, so the table separates these locations. These mixing ratios represent selected values from recent literature and are not a comprehensive listing but are meant to roughly indicate the range of levels in these regions. The reviews of Saiz-Lopez and von Glasow(30) and Saiz-Lopez and co-workers(30) present comprehensive lists of observations published before 2012, and recent observations appear in this review and in this table. More recent observations at coastal and continetal sites are described in section 3.3.1. Figure 3. Br2 production during a snow chamber experiments on 27 March 2012. Tundra snow is exposed to ambient radiation and varying ozone levels, and produced Br2 is monitored. Reprinted with permission from Pratt and co-workers (2013).(43) Copyright 2013 Nature Publishing Group. Figure 4. Evolution of dihalogen gases from laboratory experiments simulating polar halogen activation by irradiating salt-doped ice particles with or without coexposure to ozone gas. Panel a shows dihalogens (Br2 in red, Cl2 in black, and BrCl in green. Panel b shows ozone, and yellow areas indicate times when the sample was irradiated. The dotted shading visible on top of the yellow indicates when the ozone generator was switched on (dotted = ozone on, no dots = ozone off). Reprinted with permission from Wren and co-workers (2013).(110) Copyright Wren and co-workers 2013. CC Attribution 3.0 License. Figure 5. Simulated column amounts of BrO in the atmosphere versus time from the 1-D model of Toyota and co-workers (2014).(143) The panels show different assumptions for turbulence in the atmospheric boundary layer based upon windspeed, U2, and various values of the Brunt–Väisälä frequency, N. Note that the BrO column scale on each panel is different, and the higher windspeeds show much higher VCDs. Reprinted with permission from Toyota and co-workers (2014).(143) Copyright Toyota and co-workers 2014. CC Attribution 3.0 License. Figure 6. Marine boundary layer iodine monoxide (IO) observations from ship cruise and coastal station observations in pmol/mol. Reprinted with permission from Prados-Roman and co-workers (2015).(194) Copyright Prados-Roman and co-workers 2015. CC Attribution 3.0 License. Figure 7. Nightime observations of NOx polluted air containing N2O5 and produced reactive halogen precursor, nitryl chloride, ClNO2, measured at the Scripps Institution of Oceanography Pier. Reprinted with permission from Kim and co-workers (2014).(212) Copyright 2014 National Academy of Sciences. Figure 8. Conversion efficiency for production of ClNO2 from N2O5 from laboratory measurements along with models. Panel b shows ranges of particulate chloride composition from the TexAQS-GoMACCS 2006 field campaign. Reprinted with permission from Roberts and co-workers (2009).(217) Copyright 2009 American Geophysical Union. Figure 9. Mean of nightly 1 h maximum ClNO2 in January without the heterogeneous ClNO2 production (pptv = pmol/mol), (b) mean of nightly 1 h maximum ClNO2 in June without the heterogeneous ClNO2 production (pptv = pmol/mol), (c) mean of nightly 1 h maximum ClNO2 in January with the heterogeneous ClNO2 production (ppbv = nmol/mol), and (d) mean of nightly 1 h maximum ClNO2 in June with the heterogeneous ClNO2 production (ppbv = nmol/mol). Reprinted with permission from Sarwar and co-workers (2014).(222) Copyright 2014 American Geophysical Union. Figure 10. Seasonal variation of mean annual tropospheric BrO simulated by GEOS-Chem along with GOME-2 observations and p-TOMCAT model simulations. The effect of removing the heterogeneous reaction of HBr + HOBr is demonstrated in the GEOS-Chem simulations. Reprinted with permission from Parrella and co-workers (2014).(258) Copyright Parrella and co-workers 2014. CC Attribution 3.0 License. Figure 11. Modeled anthropogenic influence on oceanic iodine source as a percentage change from preindustrial times to current conditions. Reprinted with permission from Prados-Roman and co-workers.(262) Copyright Prados-Roman and co-workers 2015. CC Attribution 3.0 License. The authors declare no competing financial interest. The authors thank their respective institutions and funding agencies (National Science Foundation (US), National Aeronautics and Space Administration (US), National Oceanic and Atmospheric Administration (US), Natural Environment Research Council (UK), The Deutsche Forschungsgemeinschaft (Germany), the Spanish National Research Council, CSIC (Spain), and the European Research Council) for support in carrying out this research and review effort. We also thank an anonymous reviewer for constructive comments that improved this article. This article references 266 other publications.
中文翻译:

对流层卤素化学:来源,循环和影响
本文是
 威廉·辛普森(William Simpson)于1988年毕业于斯沃斯莫尔学院(Swarthmore College),获得了化学学士学位和数学专业的辅修学位。他于1995年在斯坦福大学获得物理化学博士学位。在两次博士后任命后,他于1997年加入阿拉斯加费尔班克斯大学,现任化学教授。他的研究小组使用光谱方法研究了大气中的卤素和氮化学,重点是北极地区以及不断变化的气候如何影响卤素的活化。
威廉·辛普森(William Simpson)于1988年毕业于斯沃斯莫尔学院(Swarthmore College),获得了化学学士学位和数学专业的辅修学位。他于1995年在斯坦福大学获得物理化学博士学位。在两次博士后任命后,他于1997年加入阿拉斯加费尔班克斯大学,现任化学教授。他的研究小组使用光谱方法研究了大气中的卤素和氮化学,重点是北极地区以及不断变化的气候如何影响卤素的活化。 史蒂文·布朗(Steven Brown)于1989年从达特茅斯学院(Dartmouth College)获得化学学士学位。随后,他在威斯康星大学麦迪逊分校获得物理化学博士学位,并于1997年进入科罗拉多州博尔德的国家海洋与大气管理局(NOAA)实验室。他目前是NOAA的研究化学家,并担任科罗拉多大学的副教授。他研究了地球大气中氮氧化物的化学性质和影响,并重点研究了对流层氮氧化物的现场测量,尤其是在黑暗中发生的那些(夜间化学反应)。他的另一个主要研究兴趣是开发用于大气和痕量气体和气溶胶颗粒的实验室和现场研究的高灵敏度光学仪器。
史蒂文·布朗(Steven Brown)于1989年从达特茅斯学院(Dartmouth College)获得化学学士学位。随后,他在威斯康星大学麦迪逊分校获得物理化学博士学位,并于1997年进入科罗拉多州博尔德的国家海洋与大气管理局(NOAA)实验室。他目前是NOAA的研究化学家,并担任科罗拉多大学的副教授。他研究了地球大气中氮氧化物的化学性质和影响,并重点研究了对流层氮氧化物的现场测量,尤其是在黑暗中发生的那些(夜间化学反应)。他的另一个主要研究兴趣是开发用于大气和痕量气体和气溶胶颗粒的实验室和现场研究的高灵敏度光学仪器。 阿方索·萨伊兹·洛佩兹(Alfonso Saiz-Lopez)在西班牙雷阿尔城学习化学。2006年,他获得了博士学位。在东英吉利大学获得大气物理化学博士学位,重点是用于大气测量和海洋边界层卤素化学的吸收光谱学。在利兹大学短暂的博士后逗留之后,他是美国喷气机推进实验室的NASA博士后学者,以及哈佛-史密森尼天体物理学中心的研究助理。自2009年以来,他是西班牙国家研究委员会(CSIC)的高级研究科学家和NCAR的附属科学家。他的小组的工作重点是大气中的卤素化学及其对气候的影响。
阿方索·萨伊兹·洛佩兹(Alfonso Saiz-Lopez)在西班牙雷阿尔城学习化学。2006年,他获得了博士学位。在东英吉利大学获得大气物理化学博士学位,重点是用于大气测量和海洋边界层卤素化学的吸收光谱学。在利兹大学短暂的博士后逗留之后,他是美国喷气机推进实验室的NASA博士后学者,以及哈佛-史密森尼天体物理学中心的研究助理。自2009年以来,他是西班牙国家研究委员会(CSIC)的高级研究科学家和NCAR的附属科学家。他的小组的工作重点是大气中的卤素化学及其对气候的影响。 乔尔·桑顿(Joel Thornton)于1996年从达特茅斯学院(Dartmouth College)获得化学学士学位。他于2002年在加州大学伯克利分校获得化学博士学位。在多伦多大学获得博士学位后,他于2004年进入华盛顿大学任教。他目前是华盛顿大学大气科学系的副教授,化学方面的会员任命。他的研究小组利用基于质谱和光谱学的最新分析技术来研究大气现象的物理化学,例如卤素化学,污染化学和气溶胶颗粒的形成/修饰。
乔尔·桑顿(Joel Thornton)于1996年从达特茅斯学院(Dartmouth College)获得化学学士学位。他于2002年在加州大学伯克利分校获得化学博士学位。在多伦多大学获得博士学位后,他于2004年进入华盛顿大学任教。他目前是华盛顿大学大气科学系的副教授,化学方面的会员任命。他的研究小组利用基于质谱和光谱学的最新分析技术来研究大气现象的物理化学,例如卤素化学,污染化学和气溶胶颗粒的形成/修饰。 罗兰·冯·格拉索(Roland von Glasow)在德国美因兹大学学习了大气物理学。然后,他完成了博士学位。2001年,在德国美因兹的马克斯-普朗克研究所(MPI)从事大气化学研究。他继续在MPI以及圣地亚哥的斯克里普斯海洋学研究所担任博士后研究员。在2007年移居英国东安格利亚大学之前,他领导了德国海德堡大学的一个研究小组三年,于2012年被提升为教授。他和他的小组开发并应用了数值模型进行研究对流层的化学和物理过程,重点是反应性卤素化学。该调查的区域包括海洋边界层,极地区域,火山羽和自由对流层。图1.卤素原子的简化反应图,在此图中以“ X”表示的是关键的化学反应路径。请注意,此图上的许多物种都是自由基,但为简单起见,只有有机自由基和有机过氧自由基,用R表示•和RO 2 •明确地显示未成对电子。图2.地球上的MODIS图像上覆盖了活性卤素物种或其前驱储层物种的主要来源。由美国国家航空航天局戈达德太空飞行中心,美国国家航空航天局可见地球项目MODIS土地小组制作的背景图片。出于篇幅考虑,仅列出了第一作者的姓氏作为参考。北半球和南半球的数据分别由NH和SH表示。如下所述,潮汐和公海区域的碘排放量有所不同,因此该表将这些位置分开。这些混合比代表了从最近文献中选择的值,并不是全面的清单,而是用来粗略地指出这些区域的含量范围。Saiz-Lopez和von Glasow(30)以及Saiz-Lopez和同事(30)的评论提供了2012年之前发表的观察的全面列表,最近的观察出现在此评论和此表中。在3.3.1节中介绍了近海和沿海地区的最新观测资料。图3. Br在2012年3月27日的雪室实验中产生了2的产量。苔原雪暴露于环境辐射和变化的臭氧水平下,并监测所产生的Br 2。未经Pratt和同事的许可转载(2013)。(43)版权所有2013 Nature Publishing Group。图4.来自实验室实验的二卤气体的演变,该实验通过在有或没有与臭氧气体共暴露的情况下照射掺盐的冰粒来模拟极性卤素的活化。小图a显示了二卤素(红色的Br 2,Cl 2黑色为BrCl,绿色为BrCl。面板b显示臭氧,黄色区域表示样品被辐照的时间。黄色上方可见的虚线阴影表示打开臭氧发生器的时间(虚线=臭氧打开,无点=臭氧关闭)。经Wren和同事的许可转载(2013)。(110)Wren和同事的版权2013。CC署名3.0许可。图5.丰田及其同事的一维模型(2014)中大气中BrO随时间变化的模拟柱量。(143)面板显示了基于风速U 2的大气边界层湍流的不同假设。,以及Brunt–Väisälä频率的各种值N。请注意,每个面板上的BrO列刻度不同,并且更高的风速显示更高的VCD。经丰田及其同事许可(2014年)转载。(143)丰田及其同事2014年版权所有。CCAttribution 3.0 License。图6.船舶巡游和海岸站观测中的海洋边界层一氧化碘(IO)观测值,以pmol / mol为单位。经Prados-Roman及其同事许可(2015)。(194)版权所有Prados-Roman及其同事2015。CCAttribution 3.0 License。图7.含有N 2 O 5和产生的活性卤素前体,硝酰氯,ClNO 2的NO x污染空气的夜间观察,由斯克里普斯海洋学码头学会(Scripps Institution of Oceanography Pier)测量。未经Kim和其同事许可转载(2014)。(212)2014年美国国家科学院版权所有。图8.实验室测量和模型从N 2 O 5产生ClNO 2的转化效率。面板b显示了TexAQS-GoMACCS 2006野外活动中氯化物颗粒组成的范围。经罗伯茨(Roberts)和同事的许可转载(2009)。(217)版权所有©美国地球物理联盟,2009年。图9.一月份没有异质ClNO 2产生的最大夜间ClNO 2的平均值(pptv = pmol / mol),(b)六月没有异质ClNO的最大夜间ClNO 2的平均值2生产(PPTV =皮摩尔/摩尔),(C)的平均的夜间1个小时最大ClNO 2月份与异质ClNO 2生产(ppbv的=纳摩尔/摩尔),和(d)平均夜间1个小时最大ClNO 2在异构ClNO 2的六月产物(ppbv = nmol / mol)。经Sarwar及其同事许可转载(2014)。(222)版权所有2014 American Earthphysical Union。图10.由GEOS-Chem模拟的年平均对流层BrO的季节变化以及GOME-2观测值和p-TOMCAT模型模拟。GEOS-Chem模拟显示了去除HBr + HOBr异质反应的效果。经Parrella及其同事许可(2014)重印。(258)Parrella及其同事2014版权所有。CCAttribution 3.0 License。图11.模拟的人为对海洋碘源的影响(从工业化前时期到当前状况的百分比变化)。经Prados-Roman及其同事许可转载。(262)版权所有Prados-Roman及其同事2015。CCAttribution 3.0许可证。作者宣称没有竞争性的经济利益。作者感谢其各自的机构和资助机构(国家科学基金会(美国),国家航空航天局(美国),国家海洋和大气管理局(美国),自然环境研究委员会(英国),德国科学基金会)(德国),西班牙国家研究委员会,CSIC(西班牙)和欧洲研究委员会)为开展这项研究和审查工作提供的支持。我们也感谢一位匿名审稿人对本文的建设性评论做出了改进。本文引用了266个其他出版物。自然环境研究委员会(英国),德国科学基金会(德国),西班牙国家研究委员会,CSIC(西班牙)和欧洲研究委员会)为开展这项研究和评估工作提供了支持。我们也感谢一位匿名审稿人对本文的建设性评论做出了改进。本文引用了266个其他出版物。自然环境研究委员会(英国),德国科学基金会(德国),西班牙国家研究委员会,CSIC(西班牙)和欧洲研究委员会)为开展这项研究和评估工作提供了支持。我们也感谢一位匿名审稿人对本文的建设性评论做出了改进。本文引用了266个其他出版物。
罗兰·冯·格拉索(Roland von Glasow)在德国美因兹大学学习了大气物理学。然后,他完成了博士学位。2001年,在德国美因兹的马克斯-普朗克研究所(MPI)从事大气化学研究。他继续在MPI以及圣地亚哥的斯克里普斯海洋学研究所担任博士后研究员。在2007年移居英国东安格利亚大学之前,他领导了德国海德堡大学的一个研究小组三年,于2012年被提升为教授。他和他的小组开发并应用了数值模型进行研究对流层的化学和物理过程,重点是反应性卤素化学。该调查的区域包括海洋边界层,极地区域,火山羽和自由对流层。图1.卤素原子的简化反应图,在此图中以“ X”表示的是关键的化学反应路径。请注意,此图上的许多物种都是自由基,但为简单起见,只有有机自由基和有机过氧自由基,用R表示•和RO 2 •明确地显示未成对电子。图2.地球上的MODIS图像上覆盖了活性卤素物种或其前驱储层物种的主要来源。由美国国家航空航天局戈达德太空飞行中心,美国国家航空航天局可见地球项目MODIS土地小组制作的背景图片。出于篇幅考虑,仅列出了第一作者的姓氏作为参考。北半球和南半球的数据分别由NH和SH表示。如下所述,潮汐和公海区域的碘排放量有所不同,因此该表将这些位置分开。这些混合比代表了从最近文献中选择的值,并不是全面的清单,而是用来粗略地指出这些区域的含量范围。Saiz-Lopez和von Glasow(30)以及Saiz-Lopez和同事(30)的评论提供了2012年之前发表的观察的全面列表,最近的观察出现在此评论和此表中。在3.3.1节中介绍了近海和沿海地区的最新观测资料。图3. Br在2012年3月27日的雪室实验中产生了2的产量。苔原雪暴露于环境辐射和变化的臭氧水平下,并监测所产生的Br 2。未经Pratt和同事的许可转载(2013)。(43)版权所有2013 Nature Publishing Group。图4.来自实验室实验的二卤气体的演变,该实验通过在有或没有与臭氧气体共暴露的情况下照射掺盐的冰粒来模拟极性卤素的活化。小图a显示了二卤素(红色的Br 2,Cl 2黑色为BrCl,绿色为BrCl。面板b显示臭氧,黄色区域表示样品被辐照的时间。黄色上方可见的虚线阴影表示打开臭氧发生器的时间(虚线=臭氧打开,无点=臭氧关闭)。经Wren和同事的许可转载(2013)。(110)Wren和同事的版权2013。CC署名3.0许可。图5.丰田及其同事的一维模型(2014)中大气中BrO随时间变化的模拟柱量。(143)面板显示了基于风速U 2的大气边界层湍流的不同假设。,以及Brunt–Väisälä频率的各种值N。请注意,每个面板上的BrO列刻度不同,并且更高的风速显示更高的VCD。经丰田及其同事许可(2014年)转载。(143)丰田及其同事2014年版权所有。CCAttribution 3.0 License。图6.船舶巡游和海岸站观测中的海洋边界层一氧化碘(IO)观测值,以pmol / mol为单位。经Prados-Roman及其同事许可(2015)。(194)版权所有Prados-Roman及其同事2015。CCAttribution 3.0 License。图7.含有N 2 O 5和产生的活性卤素前体,硝酰氯,ClNO 2的NO x污染空气的夜间观察,由斯克里普斯海洋学码头学会(Scripps Institution of Oceanography Pier)测量。未经Kim和其同事许可转载(2014)。(212)2014年美国国家科学院版权所有。图8.实验室测量和模型从N 2 O 5产生ClNO 2的转化效率。面板b显示了TexAQS-GoMACCS 2006野外活动中氯化物颗粒组成的范围。经罗伯茨(Roberts)和同事的许可转载(2009)。(217)版权所有©美国地球物理联盟,2009年。图9.一月份没有异质ClNO 2产生的最大夜间ClNO 2的平均值(pptv = pmol / mol),(b)六月没有异质ClNO的最大夜间ClNO 2的平均值2生产(PPTV =皮摩尔/摩尔),(C)的平均的夜间1个小时最大ClNO 2月份与异质ClNO 2生产(ppbv的=纳摩尔/摩尔),和(d)平均夜间1个小时最大ClNO 2在异构ClNO 2的六月产物(ppbv = nmol / mol)。经Sarwar及其同事许可转载(2014)。(222)版权所有2014 American Earthphysical Union。图10.由GEOS-Chem模拟的年平均对流层BrO的季节变化以及GOME-2观测值和p-TOMCAT模型模拟。GEOS-Chem模拟显示了去除HBr + HOBr异质反应的效果。经Parrella及其同事许可(2014)重印。(258)Parrella及其同事2014版权所有。CCAttribution 3.0 License。图11.模拟的人为对海洋碘源的影响(从工业化前时期到当前状况的百分比变化)。经Prados-Roman及其同事许可转载。(262)版权所有Prados-Roman及其同事2015。CCAttribution 3.0许可证。作者宣称没有竞争性的经济利益。作者感谢其各自的机构和资助机构(国家科学基金会(美国),国家航空航天局(美国),国家海洋和大气管理局(美国),自然环境研究委员会(英国),德国科学基金会)(德国),西班牙国家研究委员会,CSIC(西班牙)和欧洲研究委员会)为开展这项研究和审查工作提供的支持。我们也感谢一位匿名审稿人对本文的建设性评论做出了改进。本文引用了266个其他出版物。自然环境研究委员会(英国),德国科学基金会(德国),西班牙国家研究委员会,CSIC(西班牙)和欧洲研究委员会)为开展这项研究和评估工作提供了支持。我们也感谢一位匿名审稿人对本文的建设性评论做出了改进。本文引用了266个其他出版物。自然环境研究委员会(英国),德国科学基金会(德国),西班牙国家研究委员会,CSIC(西班牙)和欧洲研究委员会)为开展这项研究和评估工作提供了支持。我们也感谢一位匿名审稿人对本文的建设性评论做出了改进。本文引用了266个其他出版物。












































 京公网安备 11010802027423号
京公网安备 11010802027423号