当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic Asymmetric [4 + 2]-Cycloadditions of 2-Aminophenyl Enones with Isatin-Derived Ketimines: Diastereo- and Enantioselective Synthesis of Spirooxindole-Tetrahydroquinazolines
Organic Letters ( IF 4.9 ) Pub Date : 2023-12-26 , DOI: 10.1021/acs.orglett.3c03918
Ji Won Han 1 , Yoseop Kim 1 , Sung-Gon Kim 1
Organic Letters ( IF 4.9 ) Pub Date : 2023-12-26 , DOI: 10.1021/acs.orglett.3c03918
Ji Won Han 1 , Yoseop Kim 1 , Sung-Gon Kim 1
Affiliation
|
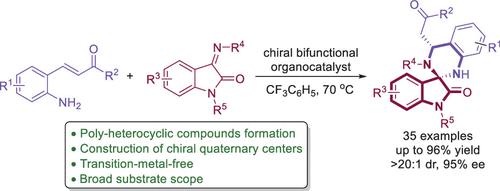
A novel method for the enantioselective synthesis of spiro N,N-heterocyclic oxindoles has been developed, employing asymmetric [4 + 2]-cycloadditions of 2-aminophenyl enones with isatin-derived ketimines. This method employs an organocatalytic approach, utilizing a bifunctional squaramide-based catalyst. It enables the precise synthesis of chiral spirooxindole-tetrahydroquinazolines with intricate structures, featuring chiral quaternary centers. This process achieves remarkable results, including high yields and exceptional levels of enantioselectivity and diastereoselectivity (up to 96% yield, 95% ee, and >20:1 dr).
中文翻译:

2-氨基苯基烯酮与靛红衍生的酮亚胺的有机催化不对称[4 + 2]-环加成:螺吲哚-四氢喹唑啉的非对映和对映选择性合成
开发了一种对映选择性合成螺N,N-杂环羟吲哚的新方法,该方法采用 2-氨基苯基烯酮与靛红衍生的酮亚胺的不对称 [4 + 2]-环加成反应。该方法采用有机催化方法,利用双功能方酰胺基催化剂。它能够精确合成具有复杂结构、具有手性四级中心的手性螺吲哚-四氢喹唑啉。该过程取得了显着的成果,包括高收率以及出色的对映选择性和非对映选择性(高达 96% 的收率、95% ee 和 >20:1 dr)。
更新日期:2023-12-26
中文翻译:

2-氨基苯基烯酮与靛红衍生的酮亚胺的有机催化不对称[4 + 2]-环加成:螺吲哚-四氢喹唑啉的非对映和对映选择性合成
开发了一种对映选择性合成螺N,N-杂环羟吲哚的新方法,该方法采用 2-氨基苯基烯酮与靛红衍生的酮亚胺的不对称 [4 + 2]-环加成反应。该方法采用有机催化方法,利用双功能方酰胺基催化剂。它能够精确合成具有复杂结构、具有手性四级中心的手性螺吲哚-四氢喹唑啉。该过程取得了显着的成果,包括高收率以及出色的对映选择性和非对映选择性(高达 96% 的收率、95% ee 和 >20:1 dr)。

































 京公网安备 11010802027423号
京公网安备 11010802027423号