当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electronic Structure and Reactivity of Mononuclear Nonheme Iron–Peroxo Complexes as a Biomimetic Model of Rieske Oxygenases: Ring Size Effects of Macrocyclic Ligands
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-12-26 , DOI: 10.1021/jacs.3c08559 Wenjuan Zhu 1 , Peng Wu 2 , Virginia A Larson 3 , Akhilesh Kumar 1 , Xiao-Xi Li 1, 4 , Mi Sook Seo 1 , Yong-Min Lee 1 , Binju Wang 5 , Nicolai Lehnert 3 , Wonwoo Nam 1, 6
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-12-26 , DOI: 10.1021/jacs.3c08559 Wenjuan Zhu 1 , Peng Wu 2 , Virginia A Larson 3 , Akhilesh Kumar 1 , Xiao-Xi Li 1, 4 , Mi Sook Seo 1 , Yong-Min Lee 1 , Binju Wang 5 , Nicolai Lehnert 3 , Wonwoo Nam 1, 6
Affiliation
|
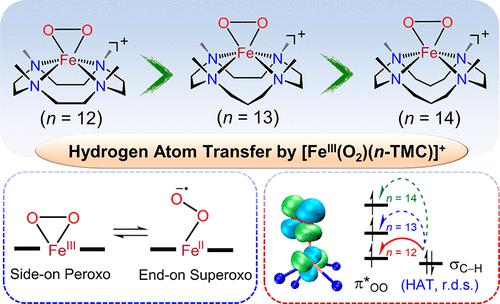
We report the macrocyclic ring size–electronic structure–electrophilic reactivity correlation of mononuclear nonheme iron(III)-peroxo complexes bearing N-tetramethylated cyclam analogues (n-TMC), [FeIII(O2)(12-TMC)]+ (1), [FeIII(O2)(13-TMC)]+ (2), and [FeIII(O2)(14-TMC)]+ (3), as a model study of Rieske oxygenases. The Fe(III)-peroxo complexes show the same δ and pseudo-σ bonds between iron and the peroxo ligand. However, the strength of these interactions varies depending on the ring size of the n-TMC ligands; the overall Fe–O bond strength and the strength of the Fe–O2 δ bond increase gradually as the ring size of the n-TMC ligands becomes smaller, such as from 14-TMC to 13-TMC to 12-TMC. MCD spectroscopy plays a key role in assigning the characteristic low-energy δ → δ* LMCT band, which provides direct insight into the strength of the Fe–O2 δ bond and which, in turn, is correlated with the superoxo character of the iron-peroxo group. In oxidation reactions, reactivities of 1–3 toward hydrocarbon C–H bond activation are compared, revealing the reactivity order of 1 > 2 > 3; the [FeIII(O2)(n-TMC)]+ complex with a smaller n-TMC ring size, 12-TMC, is much more reactive than that with a larger n-TMC ring size, 14-TMC. DFT analysis shows that the Fe(III)-peroxo complex is not reactive toward C–H bonds, but it is the end-on Fe(II)-superoxo valence tautomer that is responsible for the observed reactivity. The hydrogen atom abstraction (HAA) reactivity of these intermediates is correlated with the overall donicity of the n-TMC ligand, which modulates the energy of the singly occupied π* superoxo frontier orbital that serves as the electron acceptor in the HAA reaction. The implications of these results for the mechanism of Rieske oxygenases are further discussed.
中文翻译:

作为 Rieske 氧化酶仿生模型的单核非血红素铁过氧配合物的电子结构和反应性:大环配体的环尺寸效应
我们报道了带有N -四甲基化仙客来类似物 ( n -TMC)、[Fe III (O 2 )(12-TMC)] + ( 1 )、[Fe III (O 2 )(13-TMC)] + ( 2 ) 和 [Fe III (O 2 )(14-TMC)] + ( 3 ),作为 Rieske 加氧酶的模型研究。 Fe(III)-过氧配合物在铁和过氧配体之间显示出相同的 δ 和伪 σ 键。然而,这些相互作用的强度根据n -TMC 配体的环大小而变化;随着n -TMC配体的环尺寸变小,例如从14-TMC到13-TMC再到12-TMC,总体Fe-O键强度和Fe-O 2 δ键的强度逐渐增加。 MCD 光谱在分配特征低能 δ → δ* LMCT 能带方面发挥着关键作用,它可以直接洞察 Fe-O 2 δ 键的强度,进而与铁的超氧特性相关-过氧化基团。在氧化反应中,比较1 – 3对烃C-H键活化的反应性,揭示反应性顺序为1 > 2 > 3 ;具有较小n -TMC 环尺寸的 [Fe III (O 2 )( n -TMC)] +络合物 12-TMC 比具有较大n -TMC 环尺寸的络合物 14-TMC 更具反应性。 DFT 分析表明,Fe(III)-过氧配合物对 C-H 键不具有反应性,但末端 Fe(II)-超氧价互变异构体是造成观察到的反应性的原因。这些中间体的氢原子夺取 (HAA) 反应性与n -TMC 配体的整体单性相关,后者调节单占据 π* 超氧前沿轨道的能量,该轨道在 HAA 反应中充当电子受体。进一步讨论了这些结果对 Rieske 加氧酶机制的影响。
更新日期:2023-12-26
中文翻译:

作为 Rieske 氧化酶仿生模型的单核非血红素铁过氧配合物的电子结构和反应性:大环配体的环尺寸效应
我们报道了带有N -四甲基化仙客来类似物 ( n -TMC)、[Fe III (O 2 )(12-TMC)] + ( 1 )、[Fe III (O 2 )(13-TMC)] + ( 2 ) 和 [Fe III (O 2 )(14-TMC)] + ( 3 ),作为 Rieske 加氧酶的模型研究。 Fe(III)-过氧配合物在铁和过氧配体之间显示出相同的 δ 和伪 σ 键。然而,这些相互作用的强度根据n -TMC 配体的环大小而变化;随着n -TMC配体的环尺寸变小,例如从14-TMC到13-TMC再到12-TMC,总体Fe-O键强度和Fe-O 2 δ键的强度逐渐增加。 MCD 光谱在分配特征低能 δ → δ* LMCT 能带方面发挥着关键作用,它可以直接洞察 Fe-O 2 δ 键的强度,进而与铁的超氧特性相关-过氧化基团。在氧化反应中,比较1 – 3对烃C-H键活化的反应性,揭示反应性顺序为1 > 2 > 3 ;具有较小n -TMC 环尺寸的 [Fe III (O 2 )( n -TMC)] +络合物 12-TMC 比具有较大n -TMC 环尺寸的络合物 14-TMC 更具反应性。 DFT 分析表明,Fe(III)-过氧配合物对 C-H 键不具有反应性,但末端 Fe(II)-超氧价互变异构体是造成观察到的反应性的原因。这些中间体的氢原子夺取 (HAA) 反应性与n -TMC 配体的整体单性相关,后者调节单占据 π* 超氧前沿轨道的能量,该轨道在 HAA 反应中充当电子受体。进一步讨论了这些结果对 Rieske 加氧酶机制的影响。































 京公网安备 11010802027423号
京公网安备 11010802027423号