当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cation Chloride Cotransporter NKCC1 Operates through a Rocking-Bundle Mechanism
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-12-25 , DOI: 10.1021/jacs.3c10258 Manuel José Ruiz Munevar 1 , Valerio Rizzi 2 , Corinne Portioli 3, 4 , Pietro Vidossich 1 , Erhu Cao 5 , Michele Parrinello 6 , Laura Cancedda 4 , Marco De Vivo 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-12-25 , DOI: 10.1021/jacs.3c10258 Manuel José Ruiz Munevar 1 , Valerio Rizzi 2 , Corinne Portioli 3, 4 , Pietro Vidossich 1 , Erhu Cao 5 , Michele Parrinello 6 , Laura Cancedda 4 , Marco De Vivo 1
Affiliation
|
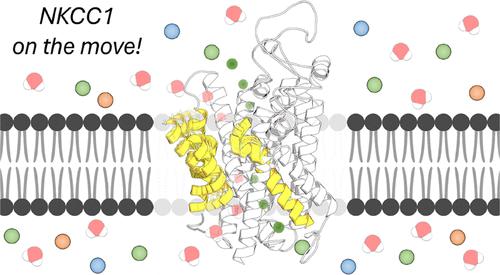
The sodium, potassium, and chloride cotransporter 1 (NKCC1) plays a key role in tightly regulating ion shuttling across cell membranes. Lately, its aberrant expression and function have been linked to numerous neurological disorders and cancers, making it a novel and highly promising pharmacological target for therapeutic interventions. A better understanding of how NKCC1 dynamically operates would therefore have broad implications for ongoing efforts toward its exploitation as a therapeutic target through its modulation. Based on recent structural data on NKCC1, we reveal conformational motions that are key to its function. Using extensive deep-learning-guided atomistic simulations of NKCC1 models embedded into the membrane, we captured complex dynamical transitions between alternate open conformations of the inner and outer vestibules of the cotransporter and demonstrated that NKCC1 has water-permeable states. We found that these previously undefined conformational transitions occur via a rocking-bundle mechanism characterized by the cooperative angular motion of transmembrane helices (TM) 4 and 9, with the contribution of the extracellular tip of TM 10. We found these motions to be critical in modulating ion transportation and in regulating NKCC1’s water transporting capabilities. Specifically, we identified interhelical dynamical contacts between TM 10 and TM 6, which we functionally validated through mutagenesis experiments of 4 new targeted NKCC1 mutants. We conclude showing that those 4 residues are highly conserved in most Na+-dependent cation chloride cotransporters (CCCs), which highlights their critical mechanistic implications, opening the way to new strategies for NKCC1’s function modulation and thus to potential drug action on selected CCCs.
中文翻译:

阳离子氯化物协同转运蛋白 NKCC1 通过摇束机制运行
钠、钾和氯协同转运蛋白 1 (NKCC1) 在严格调节离子跨细胞膜穿梭方面发挥着关键作用。最近,其异常表达和功能与许多神经系统疾病和癌症有关,使其成为治疗干预的新颖且极具前景的药理学靶点。因此,更好地了解 NKCC1 如何动态运作将对通过其调节将其开发为治疗靶点的持续努力产生广泛的影响。根据 NKCC1 的最新结构数据,我们揭示了对其功能至关重要的构象运动。通过对嵌入膜中的 NKCC1 模型进行广泛的深度学习引导的原子模拟,我们捕获了协同转运蛋白的内部和外部前庭的交替开放构象之间的复杂动态转变,并证明了 NKCC1 具有透水状态。我们发现这些先前未定义的构象转变是通过摇束机制发生的,其特征是跨膜螺旋(TM)4和9的协作角运动,以及TM 10的细胞外尖端的贡献。我们发现这些运动在调节离子运输和调节 NKCC1 的水运输能力。具体来说,我们确定了 TM 10 和 TM 6 之间的螺旋间动态接触,并通过 4 个新的靶向 NKCC1 突变体的诱变实验对其进行了功能验证。我们的结论是,这 4 个残基在大多数 Na +依赖性阳离子氯化物协同转运蛋白 (CCC) 中高度保守,这突出了它们的关键机制含义,为 NKCC1 功能调节的新策略以及对选定 CCC 的潜在药物作用开辟了道路。
更新日期:2023-12-25
中文翻译:

阳离子氯化物协同转运蛋白 NKCC1 通过摇束机制运行
钠、钾和氯协同转运蛋白 1 (NKCC1) 在严格调节离子跨细胞膜穿梭方面发挥着关键作用。最近,其异常表达和功能与许多神经系统疾病和癌症有关,使其成为治疗干预的新颖且极具前景的药理学靶点。因此,更好地了解 NKCC1 如何动态运作将对通过其调节将其开发为治疗靶点的持续努力产生广泛的影响。根据 NKCC1 的最新结构数据,我们揭示了对其功能至关重要的构象运动。通过对嵌入膜中的 NKCC1 模型进行广泛的深度学习引导的原子模拟,我们捕获了协同转运蛋白的内部和外部前庭的交替开放构象之间的复杂动态转变,并证明了 NKCC1 具有透水状态。我们发现这些先前未定义的构象转变是通过摇束机制发生的,其特征是跨膜螺旋(TM)4和9的协作角运动,以及TM 10的细胞外尖端的贡献。我们发现这些运动在调节离子运输和调节 NKCC1 的水运输能力。具体来说,我们确定了 TM 10 和 TM 6 之间的螺旋间动态接触,并通过 4 个新的靶向 NKCC1 突变体的诱变实验对其进行了功能验证。我们的结论是,这 4 个残基在大多数 Na +依赖性阳离子氯化物协同转运蛋白 (CCC) 中高度保守,这突出了它们的关键机制含义,为 NKCC1 功能调节的新策略以及对选定 CCC 的潜在药物作用开辟了道路。































 京公网安备 11010802027423号
京公网安备 11010802027423号