Materials Letters ( IF 2.7 ) Pub Date : 2023-12-26 , DOI: 10.1016/j.matlet.2023.135807
Qianwu Wang , Songlin Liu , Jingzhao Lu
|
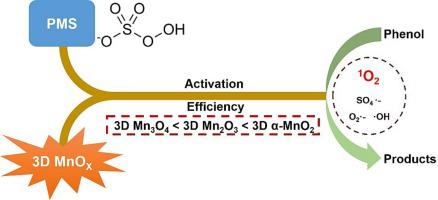
A novel three-dimensional (3D) manganese oxide (MnOX) hierarchical microflowers materials were prepared and applied to activate peroxymonosulfate (PMS) for the removal of phenol. The 3D α-MnO2 material exhibited higher catalytic activity than 3D Mn2O3 and 3D Mn3O4 materials, with 100% phenol removal within 60 min. The quenching experiment and EPR results indicated that 1O2 played a major role in removing phenol, SO4•–, O2•– and •OH ranked second. The kinetic studies indicated that the removal of phenol followed the pseudo-first-order kinetic model, and the reaction activation energy of 3D α-MnO2 material was 25.67 kJ mol−1. Besides, factors of 3D α-MnO2 catalytic performance such as catalyst dosage, PMS dose and reaction temperature were also investigated. This work provided a new approach for synthesizing 3D MnOX catalysts with high-performance and guidance for analyzing the catalytic activity of multivalent metal oxides.
中文翻译:

轻松合成三维 MnOX(MnO2、Mn2O3、Mn3O4)分级微花,通过过一硫酸盐活化去除苯酚
制备了一种新型三维(3D)氧化锰(MnO X)多级微花材料,并将其用于活化过一硫酸盐(PMS)以去除苯酚。3D α-MnO 2材料表现出比3D Mn 2 O 3和3D Mn 3 O 4材料更高的催化活性,在60 min内100%去除苯酚。猝灭实验和EPR结果表明,1 O 2对苯酚的去除起主要作用,SO 4 •–、O 2 •–和• OH 次之。动力学研究表明,苯酚的去除遵循准一级动力学模型,3D α-MnO 2材料的反应活化能为25.67 kJ mol -1。此外,还考察了催化剂用量、PMS用量和反应温度等影响3D α-MnO 2催化性能的因素。该工作为合成高性能3D MnO X催化剂提供了一种新方法,并为分析多价金属氧化物的催化活性提供了指导。

































 京公网安备 11010802027423号
京公网安备 11010802027423号