当前位置:
X-MOL 学术
›
Appl. Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of selective catalytic reduction on VOx/TiO2 catalyst by in situ ambient pressure X-ray photoelectron spectroscopy
Applied Surface Science ( IF 6.3 ) Pub Date : 2023-12-25 , DOI: 10.1016/j.apsusc.2023.159211 Yu Wang , Bin Zhou , Jingjie Guo , Yue Peng , Xu Huang , Xuefeng Chu , Wenzhe Si , Junhua Li
Applied Surface Science ( IF 6.3 ) Pub Date : 2023-12-25 , DOI: 10.1016/j.apsusc.2023.159211 Yu Wang , Bin Zhou , Jingjie Guo , Yue Peng , Xu Huang , Xuefeng Chu , Wenzhe Si , Junhua Li
|
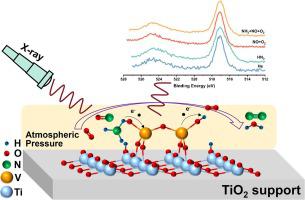
Unveiling the relationship between NH adsorption/activation behaviors and SCR reaction mechanism is imperative for rational catalyst design, but is is still not established because of lacking methodology of in situ spectroscopies. Here, vanadia-based SCR catalysts were used to identify the electron transfer and dynamic evolutions of active sites in SCR reaction by in situ AP-XPS, Raman, and DRIFTS spectra. The activation of NH occurred at VO sites, and then they reduced to VOH species by proton-coupled electron transfer process at high temperature (350 °C). Reducibility of vanadium dominated the catalytic reaction and both lattice and surface oxygen can oxidize the VOH to fulfill redox cycles. Whereas, at low temperature (150 °C), the adsorptions of NH on Brønsted acid sites was the rate-determine step of NH activations. NO was stored as nitrate/nitrite with the help of surface chemisorbed oxygen. The NH species reacted with adsorbed nitrite/nitrate to generate the NHNO intermediate that subsequently decomposed into N and HO.
中文翻译:

原位常压X射线光电子能谱选择性催化还原VOx/TiO2催化剂机理
揭示 NH 吸附/活化行为与 SCR 反应机理之间的关系对于合理的催化剂设计至关重要,但由于缺乏原位光谱学方法,目前尚未建立这种关系。在这里,基于氧化钒的 SCR 催化剂用于通过原位 AP-XPS、拉曼和 DRIFTS 光谱来识别 SCR 反应中活性位点的电子转移和动态演化。 NH 的活化发生在 VO 位点,然后在高温(350 °C)下通过质子耦合电子转移过程还原为 VOH 物质。钒的还原性主导了催化反应,晶格氧和表面氧都可以氧化VOH以完成氧化还原循环。而在低温 (150 °C) 下,NH 在布朗斯台德酸位点上的吸附是 NH 活化的速率决定步骤。在表面化学吸附氧的帮助下,NO 以硝酸盐/亚硝酸盐的形式储存。 NH 物质与吸附的亚硝酸盐/硝酸盐反应生成 NHNO 中间体,随后分解为 N 和 H2O。
更新日期:2023-12-25
中文翻译:

原位常压X射线光电子能谱选择性催化还原VOx/TiO2催化剂机理
揭示 NH 吸附/活化行为与 SCR 反应机理之间的关系对于合理的催化剂设计至关重要,但由于缺乏原位光谱学方法,目前尚未建立这种关系。在这里,基于氧化钒的 SCR 催化剂用于通过原位 AP-XPS、拉曼和 DRIFTS 光谱来识别 SCR 反应中活性位点的电子转移和动态演化。 NH 的活化发生在 VO 位点,然后在高温(350 °C)下通过质子耦合电子转移过程还原为 VOH 物质。钒的还原性主导了催化反应,晶格氧和表面氧都可以氧化VOH以完成氧化还原循环。而在低温 (150 °C) 下,NH 在布朗斯台德酸位点上的吸附是 NH 活化的速率决定步骤。在表面化学吸附氧的帮助下,NO 以硝酸盐/亚硝酸盐的形式储存。 NH 物质与吸附的亚硝酸盐/硝酸盐反应生成 NHNO 中间体,随后分解为 N 和 H2O。































 京公网安备 11010802027423号
京公网安备 11010802027423号