当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical aminotrifluoromethylation of unactivated alkenes with Langlois’ reagent as the CF3 source
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2023-12-26 , DOI: 10.1039/d3nj04705a
Tongshun An 1 , Xiaowen Qin 1 , Chenwei Liu 1 , Weiheng Yuan 1 , Tanyu Song 1 , Zhiping Yin 1
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2023-12-26 , DOI: 10.1039/d3nj04705a
Tongshun An 1 , Xiaowen Qin 1 , Chenwei Liu 1 , Weiheng Yuan 1 , Tanyu Song 1 , Zhiping Yin 1
Affiliation
|
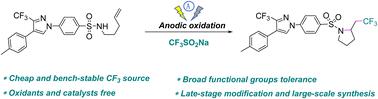
The straightforward synthesis of trifluoromethylated N-heterocycles from readily available amines has attracted significant interest from the academic community. Herein, we established a general and sustainable electrochemical approach for synthesizing various trifluoromethylated pyrrolidines. By utilizing the inexpensive and easily accessible Langlois’ reagent as the source of CF3, a broad range of unactivated alkenes was transformed into corresponding heterocycles through a trifluoromethylation/cyclization cascade process in 43–80% yields. Mechanistic investigations indicated that this reaction may be initiated with the formation of a CF3 radical through anodic single-electron transfer oxidation. The great benefit of this protocol is avoiding the use of metal catalysts and oxidants. More importantly, the late-stage modification of celecoxib and large-scale synthesis have revealed exceptionally beneficial and potential applications of the present method.
中文翻译:

以 Langlois 试剂为 CF3 源对未活化烯烃进行电化学氨基三氟甲基化
从容易获得的胺中直接合成三氟甲基化 N-杂环引起了学术界的极大兴趣。在此,我们建立了一种通用且可持续的电化学方法来合成各种三氟甲基化吡咯烷。通过利用廉价且易于获得的 Langlois' 试剂作为 CF 3的来源,通过三氟甲基化/环化级联过程将多种未活化的烯烃转化为相应的杂环,产率达到 43-80%。机理研究表明该反应可能是通过阳极单电子转移氧化形成CF 3自由基引发的。该方案的巨大好处是避免使用金属催化剂和氧化剂。更重要的是,塞来昔布的后期修饰和大规模合成揭示了本方法的异常有益和潜在的应用。
更新日期:2023-12-26
中文翻译:

以 Langlois 试剂为 CF3 源对未活化烯烃进行电化学氨基三氟甲基化
从容易获得的胺中直接合成三氟甲基化 N-杂环引起了学术界的极大兴趣。在此,我们建立了一种通用且可持续的电化学方法来合成各种三氟甲基化吡咯烷。通过利用廉价且易于获得的 Langlois' 试剂作为 CF 3的来源,通过三氟甲基化/环化级联过程将多种未活化的烯烃转化为相应的杂环,产率达到 43-80%。机理研究表明该反应可能是通过阳极单电子转移氧化形成CF 3自由基引发的。该方案的巨大好处是避免使用金属催化剂和氧化剂。更重要的是,塞来昔布的后期修饰和大规模合成揭示了本方法的异常有益和潜在的应用。































 京公网安备 11010802027423号
京公网安备 11010802027423号