Doklady Chemistry ( IF 0.8 ) Pub Date : 2023-12-25 , DOI: 10.1134/s0012500823600669 S. P. Balabanova , A. A. Voronin , A. M. Churakov , M. S. Klenov , V. A. Tartakovsky
|
Abstract
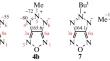
Methylation and amination reactions of 4Н-[1,2,3]triazolo[4,5-c][1,2,5]oxadiazole salts (K+, Ag+, Et3NH+, DBUH+) have been studied for the first time. It has been shown that the reaction of these salts with MeI results in two methylation products: K and Et3N salts produce 4- and 5-isomers in equal amounts, while Ag and DBU salts form mainly 4-isomer. It has been found that the main amination product of K and DBU salts of 4Н-[1,2,3]triazolo[4,5-c][1,2,5]oxadiazole with O-(p-tolylsulfonyl)hydroxylamine is 4-azido-3-amino-1,2,5-oxadiazole. A mechanism of its formation via rearrangement of 5-amino-[1,2,3]triazolo[4,5-c][1,2,5]oxadiazole has been proposed.
中文翻译:

4H-[1,2,3]三唑并[4,5-c][1,2,5]恶二唑盐的甲基化和胺化
摘要
研究了4 Н -[1,2,3]三唑并[4,5- c ][1,2,5]恶二唑盐(K +、Ag +、Et 3 NH +、DBUH + )的甲基化和胺化反应首次。已表明这些盐与MeI的反应产生两种甲基化产物:K和Et 3 N盐产生等量的4-和5-异构体,而Ag和DBU盐主要形成4-异构体。研究发现4Н-[1,2,3]三唑并[4,5-c][1,2,5]恶二唑与O-(对甲苯磺酰基)羟胺的K盐和DBU盐的主要胺化产物是4-叠氮基-3-氨基-1,2,5-恶二唑。提出了其通过 5-氨基-[1,2,3]三唑并[4,5- c ][1,2,5]恶二唑重排形成的机制。






























 京公网安备 11010802027423号
京公网安备 11010802027423号