当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Indole-6-Carboxylic Acid Derivatives as Multi-Target Antiproliferative Agents: Synthesis, in Silico Studies, and Cytotoxicity Evaluation
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2023-12-25 , DOI: 10.1002/cbdv.202301892 Mustafa M Allawi 1 , Ammar A Razzak Mahmood 2 , Lubna H Tahtamouni 3, 4 , Mai F AlSakhen 3 , Sana I Kanaan 3 , Khaled M Saleh 3 , Salem R Yasin 3
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2023-12-25 , DOI: 10.1002/cbdv.202301892 Mustafa M Allawi 1 , Ammar A Razzak Mahmood 2 , Lubna H Tahtamouni 3, 4 , Mai F AlSakhen 3 , Sana I Kanaan 3 , Khaled M Saleh 3 , Salem R Yasin 3
Affiliation
|
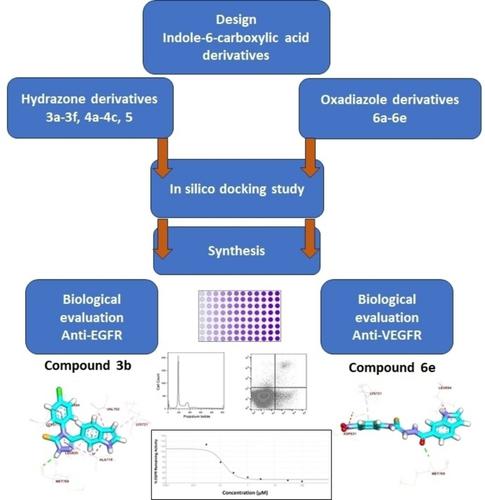
Epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR) are commonly overexpressed in cancers making them appealing targets for cancer therapeutics. Two groups of indole-6-carboxylic acid derivatives, hydrazone derivatives targeting EGFR and oxadiazole derivatives targeting VEGFR-2, were synthesized and characterized using FT-IR, 1H-NMR, 13CNMR, and HR-MS techniques. Binding patterns to potential molecular targets were studied using molecular docking and compared to standard EGFR and VEGFR-2 inhibitors. The newly synthesized compounds were cytotoxic to the three cancer cell lines tested (HCT-116, HeLa, and HT-29 cell lines) as evaluated by the MTT assay. Compound 3 b (EGFR-targeting) and compound 6 e (VEGFR-2-targeting) possessed the highest antiproliferation activity, were cancer-selective, arrested cancer cells in the G2/M phase, induced the extrinsic apoptosis pathway, and had the highest EGFR/VEGFR-2 enzyme inhibitory activity, respectively. The structure-activity relationships of the new compounds showed that the presence of an aryl or heteroaryl fragment attached to a linker is required for the anti-tumor activity. In conclusion, the findings of the current study suggest that compounds 3 b and 6 e are promising cytotoxic agents that act by inhibiting EGFR and VEGFR-2 tyrosine kinases, respectively.
中文翻译:

作为多靶点抗增殖剂的新型吲哚 6 羧酸衍生物:合成、计算机研究和细胞毒性评估
表皮生长因子受体(EGFR)和血管内皮生长因子受体(VEGFR)通常在癌症中过度表达,这使得它们成为癌症治疗的有吸引力的靶点。合成了两组吲哚-6-羧酸衍生物,即靶向EGFR的腙衍生物和靶向VEGFR-2的恶二唑衍生物,并使用FT-IR、 1 H-NMR、 13 CNMR和HR-MS技术进行表征。使用分子对接研究了与潜在分子靶标的结合模式,并与标准 EGFR 和 VEGFR-2 抑制剂进行了比较。通过 MTT 测定评估,新合成的化合物对测试的三种癌细胞系(HCT-116、HeLa 和 HT-29 细胞系)具有细胞毒性。化合物3b (靶向EGFR)和化合物6e (靶向VEGFR-2)具有最高的抗增殖活性,具有癌症选择性,将癌细胞阻滞在G2/M期,诱导外源性凋亡途径,并且具有最高的抗增殖活性。分别具有 EGFR/VEGFR-2 酶抑制活性。新化合物的结构-活性关系表明,连接基团上存在芳基或杂芳基片段是抗肿瘤活性所必需的。总之,当前研究的结果表明,化合物3b和6e是有前途的细胞毒剂,分别通过抑制 EGFR 和 VEGFR-2 酪氨酸激酶发挥作用。
更新日期:2023-12-25
中文翻译:

作为多靶点抗增殖剂的新型吲哚 6 羧酸衍生物:合成、计算机研究和细胞毒性评估
表皮生长因子受体(EGFR)和血管内皮生长因子受体(VEGFR)通常在癌症中过度表达,这使得它们成为癌症治疗的有吸引力的靶点。合成了两组吲哚-6-羧酸衍生物,即靶向EGFR的腙衍生物和靶向VEGFR-2的恶二唑衍生物,并使用FT-IR、 1 H-NMR、 13 CNMR和HR-MS技术进行表征。使用分子对接研究了与潜在分子靶标的结合模式,并与标准 EGFR 和 VEGFR-2 抑制剂进行了比较。通过 MTT 测定评估,新合成的化合物对测试的三种癌细胞系(HCT-116、HeLa 和 HT-29 细胞系)具有细胞毒性。化合物3b (靶向EGFR)和化合物6e (靶向VEGFR-2)具有最高的抗增殖活性,具有癌症选择性,将癌细胞阻滞在G2/M期,诱导外源性凋亡途径,并且具有最高的抗增殖活性。分别具有 EGFR/VEGFR-2 酶抑制活性。新化合物的结构-活性关系表明,连接基团上存在芳基或杂芳基片段是抗肿瘤活性所必需的。总之,当前研究的结果表明,化合物3b和6e是有前途的细胞毒剂,分别通过抑制 EGFR 和 VEGFR-2 酪氨酸激酶发挥作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号