Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2023-12-25 , DOI: 10.1016/j.aca.2023.342175 Yufei Ma 1 , Yuhan Xiang 1 , Xin Li 2 , Dandan Zhang 3 , Qing Chen 1
|
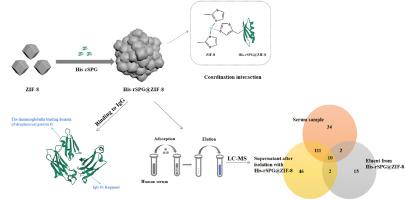
A novel solid phase extractant His-rSPG@ZIF-8 was prepared by covalently coupling recombinant streptococcal protein G (His-rSPG) with ZIF-8. The His-rSPG@ZIF-8 composite was characterized by Fourier transform infrared spectroscopy (FT-IR), Raman spectroscopy (Raman), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Due to the specific binding between the immunoglobulin binding region of His-rSPG and the Fc region of immunoglobulin G (IgG), the His-rSPG@ZIF-8 composite demonstrated exceptional selectivity in adsorbing IgG. In Britton-Robinson buffer (BR buffer) with a salt concentration of 500 mmol L−1 (0.04 mol L−1, pH 8.0), the His-rSPG@ZIF-8 composite exhibited a remarkable adsorption efficiency of 99.8 % for 0.05 mg of the composite on 200 μL of IgG solution (100 μg mL−1). The adsorption behavior of the His-rSPG@ZIF-8 composite aligns with the Langmuir adsorption model, and the theoretical maximum adsorption capacity is 1428.6 mg g−1. The adsorbed IgG molecules were successfully eluted using a SDS solution (0.5 %, m/m), resulting in a recovery rate of 91.2 %. Indeed, the His-rSPG@ZIF-8 composite was successfully utilized for the isolation and purification of IgG from human serum samples. The obtained IgG exhibited high purity, as confirmed by SDS-PAGE analysis. Additionally, LC-MS/MS analysis was employed to identify the human serum proteins following the adsorption and elution process using the His-rSPG@ZIF-8 composite material. The results revealed that the recovered solution contained an impressive content of immunoglobulin, accounting for 62.4 % of the total protein content. Furthermore, this process also led to the significant enrichment of low abundance proteins such as Serpin B4 and Cofilin-1. Consequently, the His-rSPG@ZIF-8 composite holds great promise for applications such as IgG purification and immunoassays. At the same time, it expands the application of metal-organic frameworks in the field of proteomics.
中文翻译:

重组链球菌蛋白 G 修饰的金属有机框架 ZIF-8 用于从人血清中高度选择性纯化免疫球蛋白 G
通过重组链球菌G蛋白(His-rSPG)与ZIF-8共价偶联,制备了新型固相提取剂His-rSPG@ZIF-8。 His-rSPG@ZIF-8复合材料通过傅里叶变换红外光谱(FT-IR)、拉曼光谱(Raman)、X射线光电子能谱(XPS)、扫描电子显微镜(SEM)和透射电子显微镜(TEM)进行表征。由于His-rSPG的免疫球蛋白结合区与免疫球蛋白G (IgG)的Fc区之间的特异性结合,His-rSPG@ZIF-8复合物在吸附IgG方面表现出优异的选择性。在盐浓度为500 mmol L -1 (0.04 mol L -1 , pH 8.0)的Britton-Robinson缓冲液(BR缓冲液)中,His-rSPG@ZIF-8复合材料对0.05 mg的吸附效率高达99.8 %复合物在200μL IgG溶液(100μgmL -1 )上的分析。 His-rSPG@ZIF-8复合材料的吸附行为符合Langmuir吸附模型,理论最大吸附容量为1428.6 mg g -1 。使用 SDS 溶液(0.5%,m/m)成功洗脱吸附的 IgG 分子,回收率为 91.2%。事实上,His-rSPG@ZIF-8 复合材料已成功用于从人血清样品中分离和纯化 IgG。通过 SDS-PAGE 分析证实,获得的 IgG 具有高纯度。此外,使用 His-rSPG@ZIF-8 复合材料进行吸附和洗脱过程后,采用 LC-MS/MS 分析来鉴定人血清蛋白。结果显示,回收液中免疫球蛋白含量惊人,占总蛋白含量的62.4%。 此外,这个过程还导致低丰度蛋白质(例如 Serpin B4 和 Cofilin-1)的显着富集。因此,His-rSPG@ZIF-8 复合材料在 IgG 纯化和免疫分析等应用中具有广阔的前景。同时,拓展了金属有机框架在蛋白质组学领域的应用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号