Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2023-12-24 , DOI: 10.1016/j.bmc.2023.117578 Yu Jung Park 1 , Hee Jin Jung 1 , Hye Jin Kim 1 , Hye Soo Park 1 , Jieun Lee 1 , Dahye Yoon 1 , Min Kyung Kang 1 , Ga Young Kim 1 , Sultan Ullah 2 , Dongwan Kang 3 , Yujin Park 3 , Pusoon Chun 4 , Hae Young Chung 5 , Hyung Ryong Moon 1
|
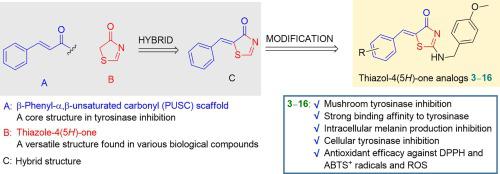
As the β-phenyl-α,β-unsaturated carbonyl (PUSC) structure was previously identified to play a key role in tyrosinase inhibition, 14 analogs with a PUSC structure built on a thiazol-4(5H)-one scaffold were synthesized using Knoevenagel condensation to serve as potential tyrosinase inhibitors. Through mushroom tyrosinase inhibition experiments, two analogs 9 and 11 were identified as potent tyrosinase inhibitors, with 11 exhibiting an IC50 value of 0.4 ± 0.01 μM, which indicates its 26-fold greater potency than kojic acid. Kinetic studies using Lineweaver-Burk plots revealed that 9 and 11 are competitive and mixed-type inhibitors, respectively; these kinetic results were supported by docking simulations. According to the B16F10 cell-based experiments, 9 and 11 inhibited melanogenesis more effectively than kojic acid due to their potent cellular tyrosinase inhibitory activity. In addition, analogs 9 and 11 exhibited moderate-to-strong antioxidant capacity, scavenging ABTS+, DPPH, and ROS radicals. In particular, analog 12 with a catechol moiety exhibited very strong ROS-scavenging activity, similar to Trolox. These results suggest that analogs 9 and 11, which exhibit potent tyrosinase inhibitory activity in mushroom and mammalian cells and anti-melanogenic effects in B16F10 cells, are promising antibrowning agents for crops and skin lightening agents for hyperpigmentation-related diseases.
中文翻译:

Thiazol-4(5H)-one 类似物作为有效的酪氨酸酶抑制剂:合成、酪氨酸酶抑制、抗黑色素生成作用、抗氧化活性和计算机对接模拟
由于 β-苯基-α,β-不饱和羰基 (PUSC) 结构先前已被确定在酪氨酸酶抑制中发挥关键作用,因此使用 thiazol-4(5 H )-one 支架合成了 14 种具有 PUSC 结构的类似物Knoevenagel 缩合可作为潜在的酪氨酸酶抑制剂。通过蘑菇酪氨酸酶抑制实验,两种类似物9和11被鉴定为有效的酪氨酸酶抑制剂,其中11的IC 50值为0.4 ± 0.01 μM,这表明其效力比曲酸高26倍。使用 Lineweaver-Burk 图进行的动力学研究表明, 9和11分别是竞争型和混合型抑制剂;这些动力学结果得到了对接模拟的支持。根据 B16F10 细胞实验, 9和11由于具有强大的细胞酪氨酸酶抑制活性,比曲酸更有效地抑制黑色素生成。此外,类似物9和11表现出中等到强的抗氧化能力,清除ABTS + 、DPPH和ROS自由基。特别是,带有儿茶酚部分的类似物12表现出非常强的 ROS 清除活性,类似于 Trolox。这些结果表明,类似物9和11在蘑菇和哺乳动物细胞中表现出有效的酪氨酸酶抑制活性,并且在B16F10细胞中表现出抗黑色素生成作用,是有希望的农作物抗褐变剂和用于色素沉着过度相关疾病的皮肤美白剂。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号