Separation and Purification Technology ( IF 8.1 ) Pub Date : 2023-12-23 , DOI: 10.1016/j.seppur.2023.126162
Junyuan Hua , Jintao He , Hongchang Pei , Jiahui Du , Xiaohua Ma , Jianxin Li
|
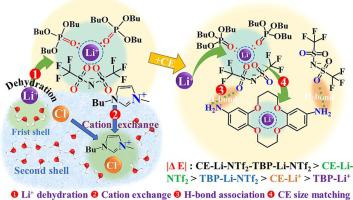
The high similarity in chemical properties between Li+ and Mg2+ poses a substantial challenge for their selective separation from salt lake brine with high Mg-Li (m/m) ratios. Herein, a highly efficient solvent extraction system was reported for Li+/Mg2+ selective separation, with tributyl phosphate (TBP) and di(aminobenzo)-14-crown-4 (CE) as extractants, [C4mim][NTf2] (IL) as co-extractant, and methylene chloride as a diluent. Simulated brines included 0.01 M LiCl and 0.01 and 0.2 M MgCl2 with Mg/Li mass ratios of 3.5 and 70, respectively. Molecular dynamics (MD), density functional theory (DFT), and 1H NMR analysis were employed to reveal the hydrogen bond interaction and compatibility between CE, TBP, and IL. Under the optimal extraction conditions containing a concentration of 20 mg/mL CE and 0.61 mmol/mL IL, the maximum single-stage Li+ extraction rate of the TBP-CE-IL extraction system with Mg/Li mass ratios of 3.5 and 70 were 89.5 and 81.8 %, and the separation factor of Li to Mg were up to 515 and 422. Compared with the TBP-IL extraction system, the addition of CE effectively improved the extraction rate of Li+ and the selectivity of Mg-Li, which was due to the hydrogen bond interaction between CE and TBP/IL and the cavity size effect between CE and Li+, improving CE coordination and endowing the complex generated in the organic phase with greater stability. The possible structure of the complex in the organic phase was determined to be 2TBP·Li·NTf2-CE·Li·NTf2 through Li+ extraction dynamic analysis. In summary, this work provides an efficient separation technology for Li+ extraction from salt lake brine.
中文翻译:

在磷酸三丁酯-离子液体萃取体系中添加冠醚作为共萃取剂,同时显着提高Li+/Mg2+选择性和Li+回收率
Li +和Mg 2+化学性质高度相似,这对从高Mg-Li (m/m)比盐湖卤水中选择性分离它们提出了重大挑战。在此,报道了一种用于Li + /Mg 2+选择性分离的高效溶剂萃取系统,以磷酸三丁酯(TBP)和二(氨基苯并)-14-crown-4(CE)作为萃取剂,[C 4 mim][NTf 2 ](IL)作为共萃取剂,二氯甲烷作为稀释剂。模拟盐水包括0.01 M LiCl 以及0.01 和0.2 M MgCl 2,Mg/Li 质量比分别为3.5 和70。采用分子动力学 (MD)、密度泛函理论 (DFT) 和1 H NMR 分析揭示了CE、TBP 和 IL 之间的氢键相互作用和相容性。在20 mg/mL CE和0.61 mmol/mL IL的最佳萃取条件下, Mg/Li质量比为3.5和70的TBP-CE-IL萃取体系的单级Li +萃取率最大为89.5%和81.8%,Li与Mg的分离因子分别达到515和422。与TBP-IL萃取体系相比,CE的加入有效提高了Li +的萃取率和Mg-Li的选择性,由于CE与TBP/IL之间的氢键相互作用以及CE与Li +之间的空腔尺寸效应,改善了CE配位并使有机相中生成的络合物具有更大的稳定性。通过Li +萃取动力学分析,确定有机相中配合物的可能结构为2TBP·Li·NTf 2 -CE·Li·NTf 2。综上所述,本工作为盐湖卤水提取Li +提供了一种高效的分离技术。

































 京公网安备 11010802027423号
京公网安备 11010802027423号